20th Annual Global Investment Conference, sponsored by H.C. Wainwright September 4-6, 2018 1

20th Annual Global Investment Conference, sponsored by H.C. Wainwright September 4-6, 2018 1

Forward-Looking Statements This presentation may include forward-looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts. Such forward-looking statements are subject to significant risks and uncertainties that are subject to change based on various factors (many of which are beyond Cerecor’s control), which could cause actual results to differ from the forward-looking statements. Such statements may include, without limitation, statements with respect to Cerecor’s plans, objectives, projections, expectations and intentions and other statements identified by words such as “projects,” “may,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “aims,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” or similar expressions (including their use in the negative), or by discussions of future matters such as: our 2018 outlook; the development of product candidates or products; potential attributes and benefits of product candidates; the expansion of Cerecor’s drug portfolio, Cerecor’s ability to identify new indications for its current portfolio; and new product candidates that could be in-licensed, and other statements that are not historical. These statements are based upon the current beliefs and expectations of Cerecor’s management but are subject to significant risks and uncertainties, including: risks associated with acquisitions, including the need to quickly and successfully integrate acquired assets and personnel; Cerecor’s cash position and the potential need for it to raise additional capital; reliance on key personnel, including Mr. Greenleaf; drug development costs and timing; and those other risks detailed in Cerecor’s filings with the Securities and Exchange Commission. Actual results may differ from those set forth in the forward-looking statements. Except as required by applicable law, Cerecor expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Cerecor’s expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based. 2 Cerecor Inc.

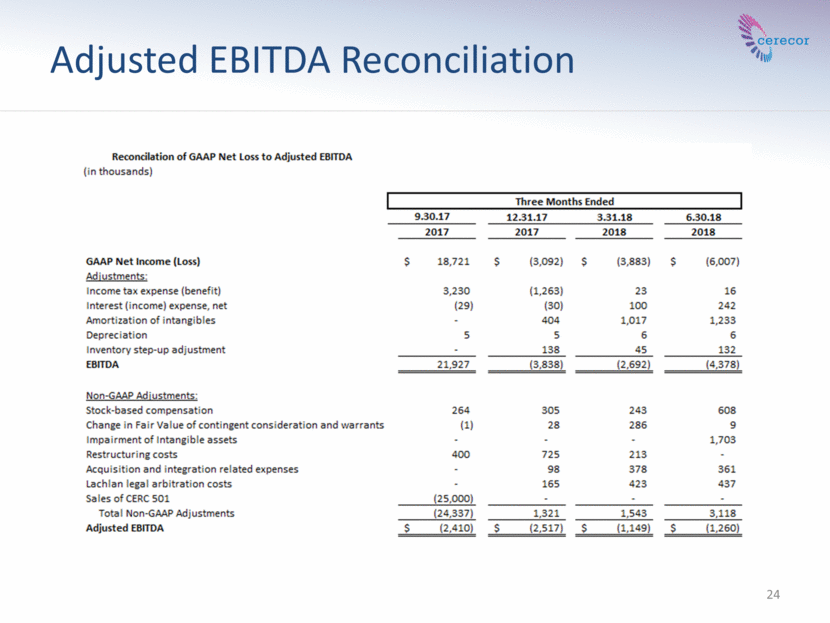
Non-GAAP Financial Measures This presentation contains two financial metrics (EBITDA and Adjusted EBITDA) that are considered “non-GAAP” financial metrics under applicable Securities and Exchange Commission rules and regulations. These non-GAAP financial metrics should be considered supplemental to and not a substitute for financial information prepared in accordance with generally accepted accounting principles. The Company’s definition of these non-GAAP metrics may differ from similarly titled metrics used by companies. EBITDA reflects GAAP net income adjusted to exclude (i) taxes, (ii) interest expense, (iii) interest income, (iv) amortization of intangibles, (v) depreciation, and (vi) inventory step-up adjustment recognized in earnings. Adjusted EBITDA adjusts EBITDA for specified items that can be highly variable or difficult to predict, and various non-cash items, including (i) share-based compensation expense, (ii) change in fair value of contingent consideration, warrant liability and unit purchase option liability (iii) one-time severance payments, (iv) restructuring costs, (v) acquisition and integration-related expenses, (vi) impairment of intangible assets, (vii) arbitration costs related to the Lachlan transaction, and (viii) sale of CERC 501. The Company views these non-GAAP financial metrics as a means to facilitate management’s financial and operational decision-making, including evaluation of the Company’s historical operating results and comparison to competitors’ operating results. These non-GAAP financial metrics reflect an additional way of viewing aspects of the Company’s operations that, when viewed with GAAP results, may provide a more complete understanding of factors and trends affecting the Company’s business. The determination of the amounts that are excluded from these non-GAAP financial metrics is a matter of management judgment and depends upon, among other factors, the nature of the underlying expense or income amounts. Because non-GAAP financial metrics exclude the effect of items that will increase or decrease the Company’s reported results of operations, management strongly encourages investors to review the Company’s consolidated financial statements and publicly-filed reports in their entirety. 3 Cerecor Inc.

Dedicated to commercializing, acquiring, and developing innovative treatments that make a difference in the lives of children 4

while building a robust pipeline of orphan therapies for future growth. 5
Overview 6 Experienced Management Team Historical Milestones Pediatric Portfolio Pediatric, Neurology & Rare Diseases Pipeline Strategic Growth Plans and Outlook Financial Highlights 1 2 3 4 5 6

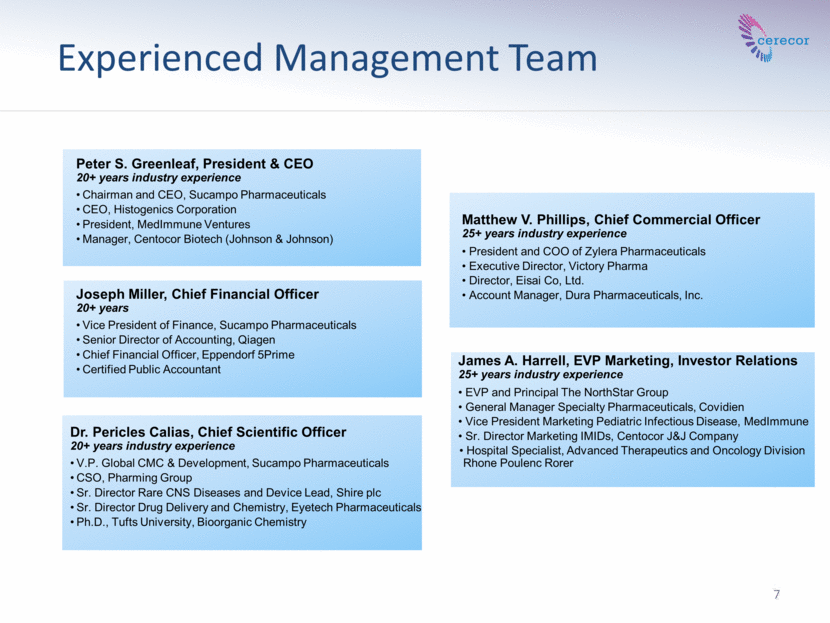
Experienced Management Team 7 Peter S. Greenleaf, President & CEO 20+ years industry experience Chairman and CEO, Sucampo Pharmaceuticals CEO, Histogenics Corporation President, MedImmune Ventures Manager, Centocor Biotech (Johnson & Johnson) Dr. Pericles Calias, Chief Scientific Officer 20+ years industry experience V.P. Global CMC & Development, Sucampo Pharmaceuticals CSO, Pharming Group Sr. Director Rare CNS Diseases and Device Lead, Shire plc Sr. Director Drug Delivery and Chemistry, Eyetech Pharmaceuticals Ph.D., Tufts University, Bioorganic Chemistry Matthew V. Phillips, Chief Commercial Officer 25+ years industry experience President and COO of Zylera Pharmaceuticals Executive Director, Victory Pharma Director, Eisai Co, Ltd. Account Manager, Dura Pharmaceuticals, Inc. James A. Harrell, EVP Marketing, Investor Relations 25+ years industry experience EVP and Principal The NorthStar Group General Manager Specialty Pharmaceuticals, Covidien Vice President Marketing Pediatric Infectious Disease, MedImmune Sr. Director Marketing IMIDs, Centocor J&J Company Hospital Specialist, Advanced Therapeutics and Oncology Division Rhone Poulenc Rorer Joseph Miller, Chief Financial Officer 20+ years Vice President of Finance, Sucampo Pharmaceuticals Senior Director of Accounting, Qiagen Chief Financial Officer, Eppendorf 5Prime Certified Public Accountant
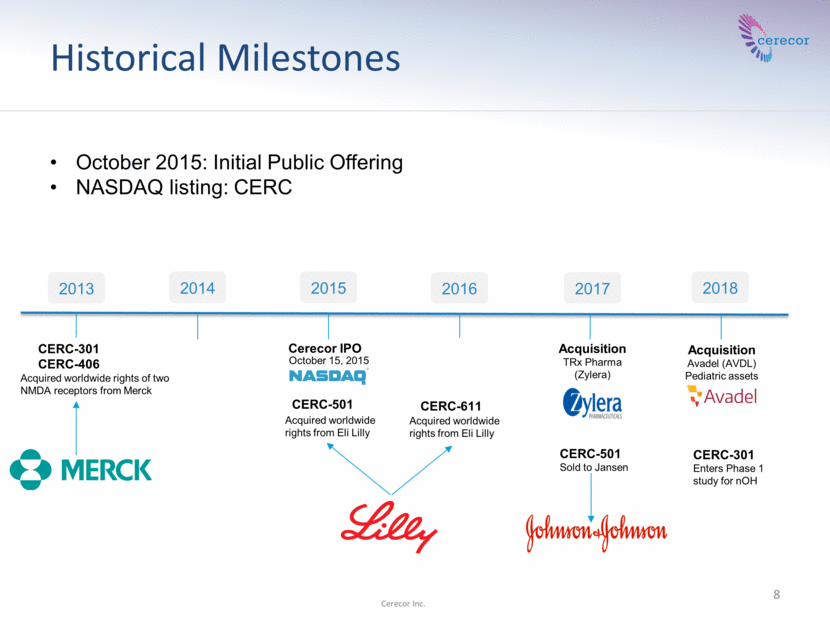
Historical Milestones 8 Cerecor Inc. 2017 2013 2016 2015 2014 CERC-301 CERC-406 CERC-611 CERC-501 Sold to Jansen CERC-501 2018 CERC-301 Enters Phase 1 study for nOH October 2015: Initial Public Offering NASDAQ listing: CERC Acquisition TRx Pharma (Zylera) Acquisition Avadel (AVDL) Pediatric assets Acquired worldwide rights of two NMDA receptors from Merck Acquired worldwide rights from Eli Lilly Acquired worldwide rights from Eli Lilly Cerecor IPO October 15, 2015
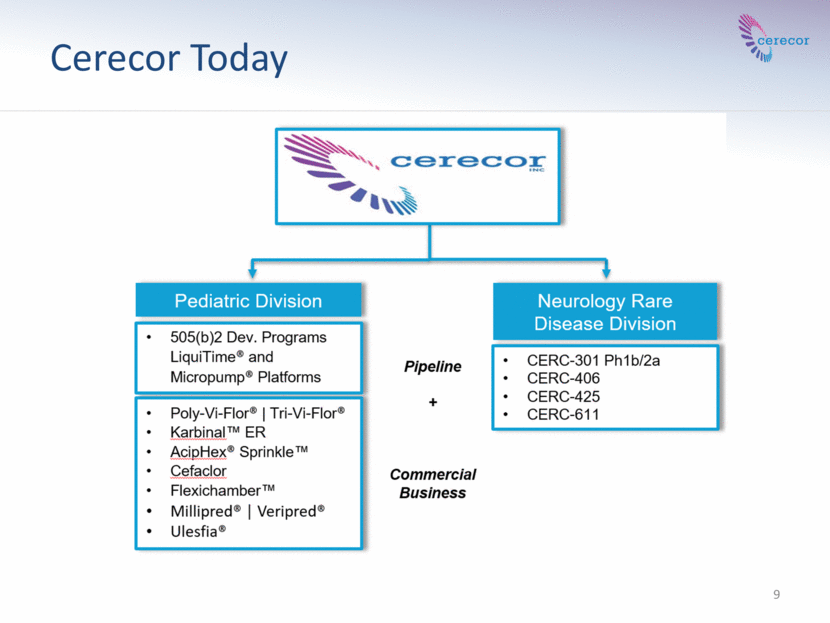
Cerecor Today 9
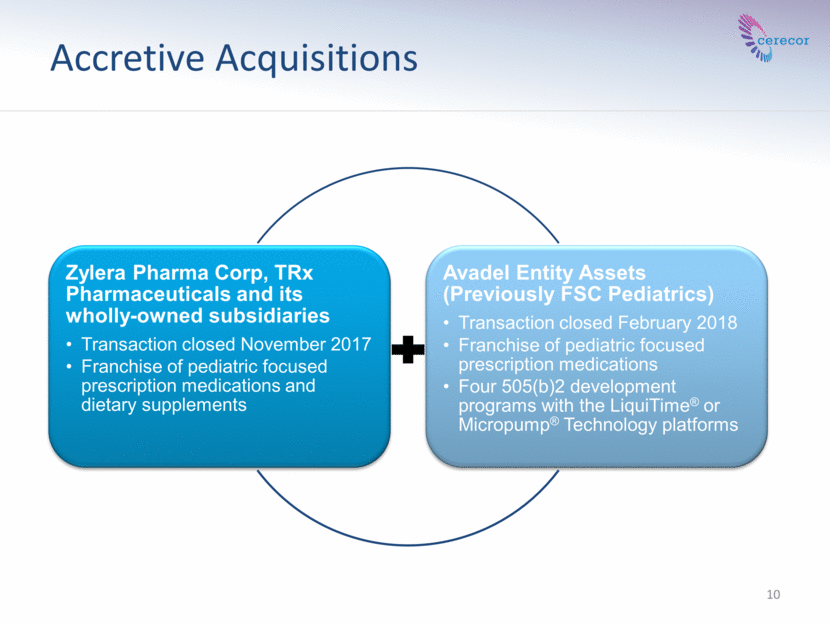
Accretive Acquisitions 10 Zylera Pharma Corp, TRx Pharmaceuticals and its wholly-owned subsidiaries Transaction closed November 2017 Franchise of pediatric focused prescription medications and dietary supplements Avadel Entity Assets (Previously FSC Pediatrics) Transaction closed February 2018 Franchise of pediatric focused prescription medications Four 505(b)2 development programs with the LiquiTime® or Micropump® Technology platforms
Pediatric Franchise with Eight Product Lines 11 Cerecor Inc. TRX Pharmaceuticals Avadel Pediatric Assets

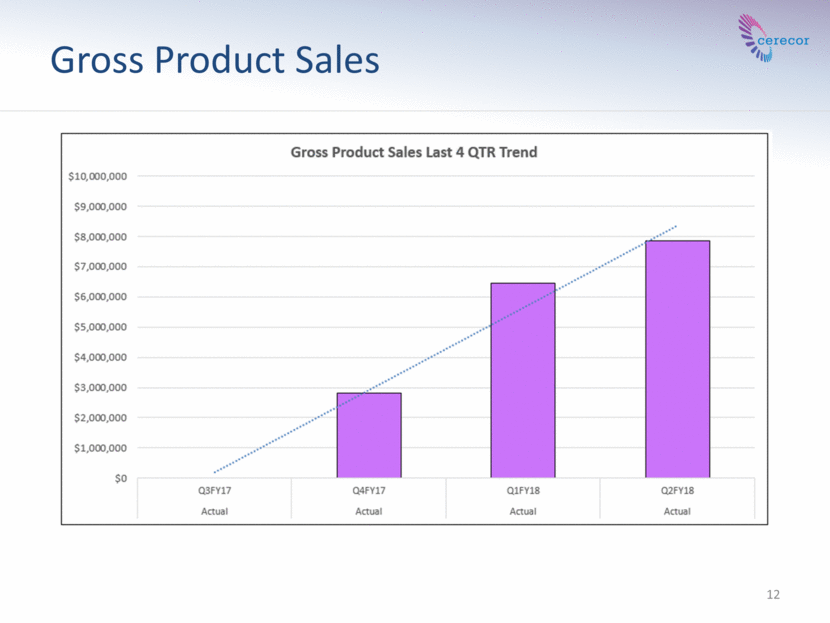
12 Gross Product Sales
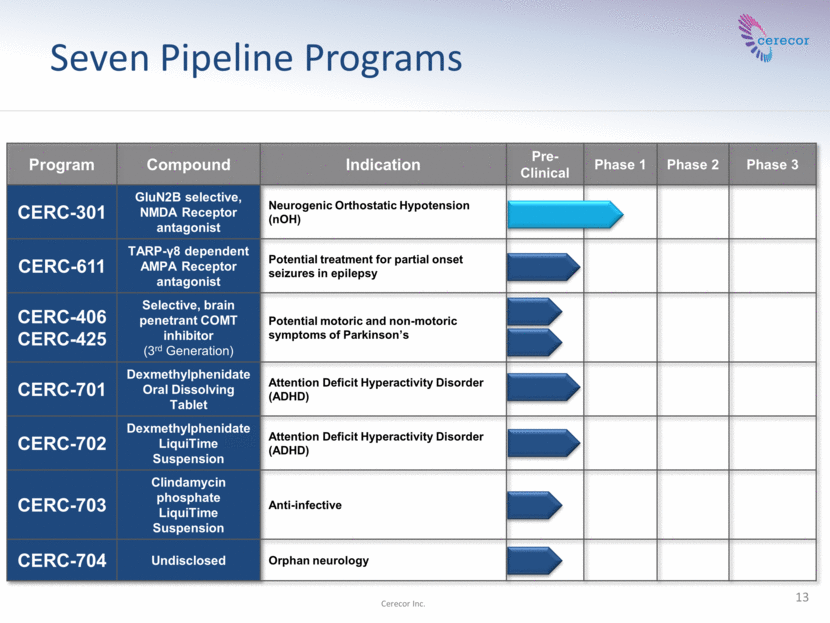
Seven Pipeline Programs Program Compound Indication Pre-Clinical Phase 1 Phase 2 Phase 3 CERC-301 GluN2B selective, NMDA Receptor antagonist Neurogenic Orthostatic Hypotension (nOH) CERC-611 TARP-γ8 dependent AMPA Receptor antagonist Potential treatment for partial onset seizures in epilepsy CERC-406 CERC-425 Selective, brain penetrant COMT inhibitor (3rd Generation) Potential motoric and non-motoric symptoms of Parkinson’s CERC-701 Dexmethylphenidate Oral Dissolving Tablet Attention Deficit Hyperactivity Disorder (ADHD) CERC-702 Dexmethylphenidate LiquiTime Suspension Attention Deficit Hyperactivity Disorder (ADHD) CERC-703 Clindamycin phosphate LiquiTime Suspension Anti-infective CERC-704 Undisclosed Orphan neurology 13 Cerecor Inc.
Exclusive Licensing Deal with Avadel Cerecor and Avadel have an exclusive licensing deal for up to four 505(b)2 assets to be developed using the Micropump® and LiquiTime® technology 14 Micropump® Micropump® is a microparticulate system that allows the development of modified and/or controlled release of solid, oral dosage formulations of drugs in a variety of formats (such as tablets, capsules, sachet, or liquids LiquiTime® Employs the Micropump® technology to allow development of modified/controlled release liquid suspension formulations Focus on products particularly suitable for dosing children and other patients having issues swallowing tablets or capsules Advantages of LiquiTime®-developed products: Easy-to-swallow formulations Good mouthfeel Taste-masked Dosing flexibility
CERC-700 Series On-going Programs ADHD CERC-701: Dexmethylphenidate delayed release, rapid dissolving tablet formulation CERC-702: Dexmethylphenidate immediate release, taste masked extended release liquid formulation Anti-Infective CERC-703: Clindamycin hydrochloride, liquid and extended release Expands our existing in-line Pediatric Franchise with additional products 15

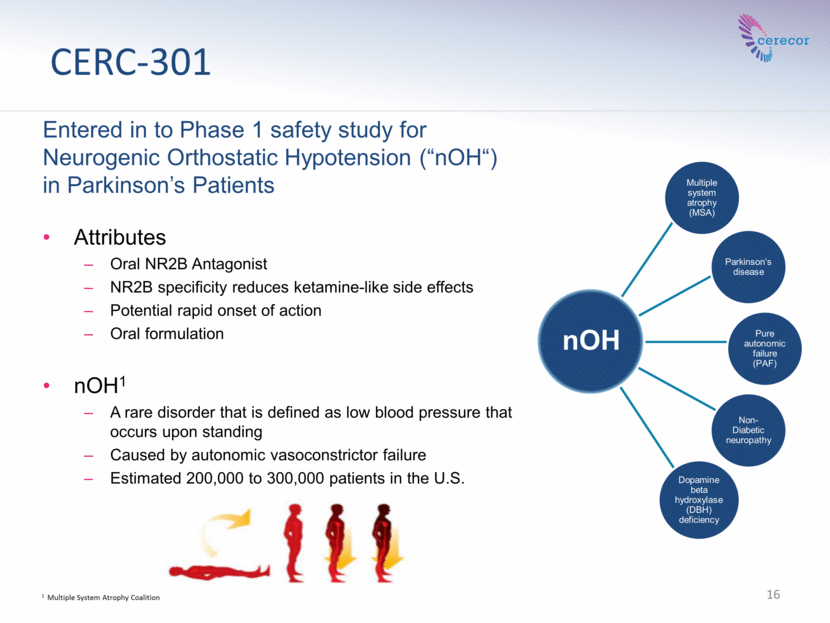
CERC-301 Entered in to Phase 1 safety study for Neurogenic Orthostatic Hypotension (“nOH“) in Parkinson’s Patients Attributes Oral NR2B Antagonist NR2B specificity reduces ketamine-like side effects Potential rapid onset of action Oral formulation nOH1 A rare disorder that is defined as low blood pressure that occurs upon standing Caused by autonomic vasoconstrictor failure Estimated 200,000 to 300,000 patients in the U.S. 16 1 Multiple System Atrophy Coalition Multiple system atrophy (MSA) Parkinson’s disease Pure autonomic failure (PAF) Non-Diabetic neuropathy Dopamine beta hydroxylase (DBH) deficiency nOH
CERC-611 Significant unmet need Epilepsy affects over 65 million patients worldwide 30%-40% of patients refractory; high degree of poly-pharmacy common All anti-seizure drugs have side effects (e.g. motoric) limiting use and the timely achievement of therapeutic dose levels Unique Mechanism of Action CERC-611 is the first known AMPA receptor antagonist that selectively targets the hippocampus AMPA receptors mediate fast synaptic neurotransmission within the CNS and are a proven target for anti-seizure efficacy CERC-611 shows lack of motoric impairment at efficacious exposures in animal models, e.g. cerebellum sparing 17 A preclinical / Phase 1-ready candidate with therapeutic potential for partial onset seizures in patients with epilepsy
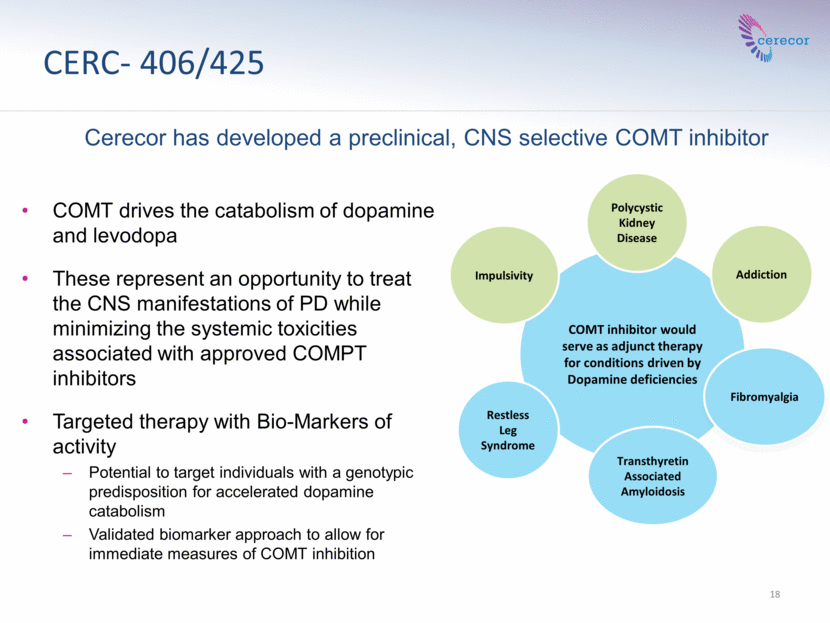
CERC- 406/425 COMT drives the catabolism of dopamine and levodopa These represent an opportunity to treat the CNS manifestations of PD while minimizing the systemic toxicities associated with approved COMPT inhibitors Targeted therapy with Bio-Markers of activity Potential to target individuals with a genotypic predisposition for accelerated dopamine catabolism Validated biomarker approach to allow for immediate measures of COMT inhibition 18 COMT inhibitor would serve as adjunct therapy for conditions driven by Dopamine deficiencies Impulsivity Addiction Polycystic Kidney Disease Restless Leg Syndrome Transthyretin Associated Amyloidosis Fibromyalgia Cerecor has developed a preclinical, CNS selective COMT inhibitor
2018 Growth Plans 19 Cerecor Inc. Develop Commercial Excellence Accelerate Business Development Activity Advance Pipeline Grow Market Share Expand Commercial Footprint Acquire/in-license early and late stage commercial-ready or marketed asset(s) Advance CERC -700’s Advance CERC-301 Progress CERC-611 Target ID 406/425 1 3 2

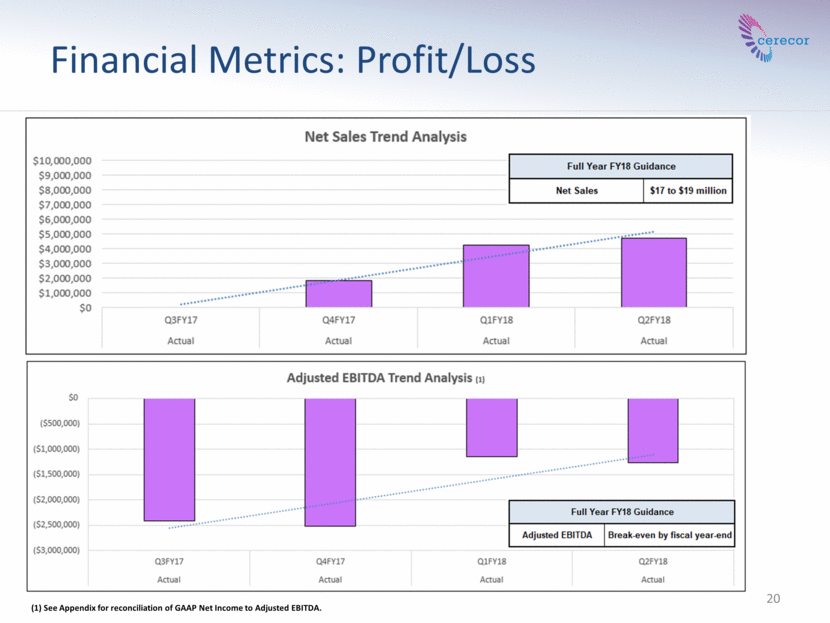
20 Financial Metrics: Profit/Loss (1) See Appendix for reconciliation of GAAP Net Income to Adjusted EBITDA.
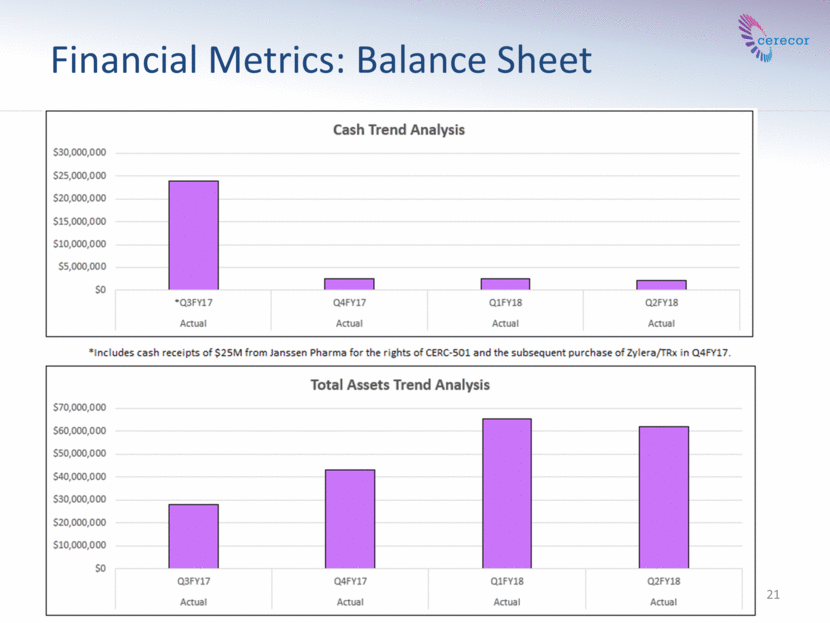
21 Financial Metrics: Balance Sheet
Cerecor Inc. NASDAQ:CERC www.cerecor.com 22

Appendix 23

24 Adjusted EBITDA Reconciliation