
July | 2021 Establishing a leading, rare and orphan disease-focused biopharmaceutical company to deliver impactful new medicines to patients Patient-Inspired Science Exhibit 99.1

2 | Forward-Looking Statements This presentation may include forward-looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts. Such forward-looking statements are subject to significant risks and uncertainties that are subject to change based on various factors (many of which are beyond the control of Cerecor Inc. (“Cerecor” or the “Company”), which could cause actual results to differ from the forward-looking statements. Such statements may include, without limitation, statements with respect to Cerecor’s plans, objectives, projections, expectations and intentions and other statements identified by words such as “projects,” “may,” “might,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “aims,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” or similar expressions (including their use in the negative), or by discussions of future matters such as: its future financial and operational outlook; the development of product candidates or products; potential attributes and benefits of product candidates; strategic alternatives for neurological assets and Millipred; and other statements that are not historical. These statements are based upon the current beliefs and expectations of Cerecor’s management but are subject to significant risks and uncertainties, including: reliance on and integration of key personnel; drug development costs, timing and other risks, including reliance on investigators and enrollment of patients in clinical trials, which might be slowed by the COVID-19 pandemic; regulatory risks; Cerecor’s cash position and the need for it to raise additional capital; risks related to potential strategic alternatives for its neurology assets and Millipred; and general economic and market risks and uncertainties, including those caused by the COVID-19 pandemic and those other risks detailed in Cerecor’s filings with the Securities and Exchange Commission. Actual results may differ from those set forth in the forward-looking statements. Except as required by applicable law, Cerecor expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Cerecor’s expectations with respect thereto or any change in events, conditions, or circumstances on which any statement is based.
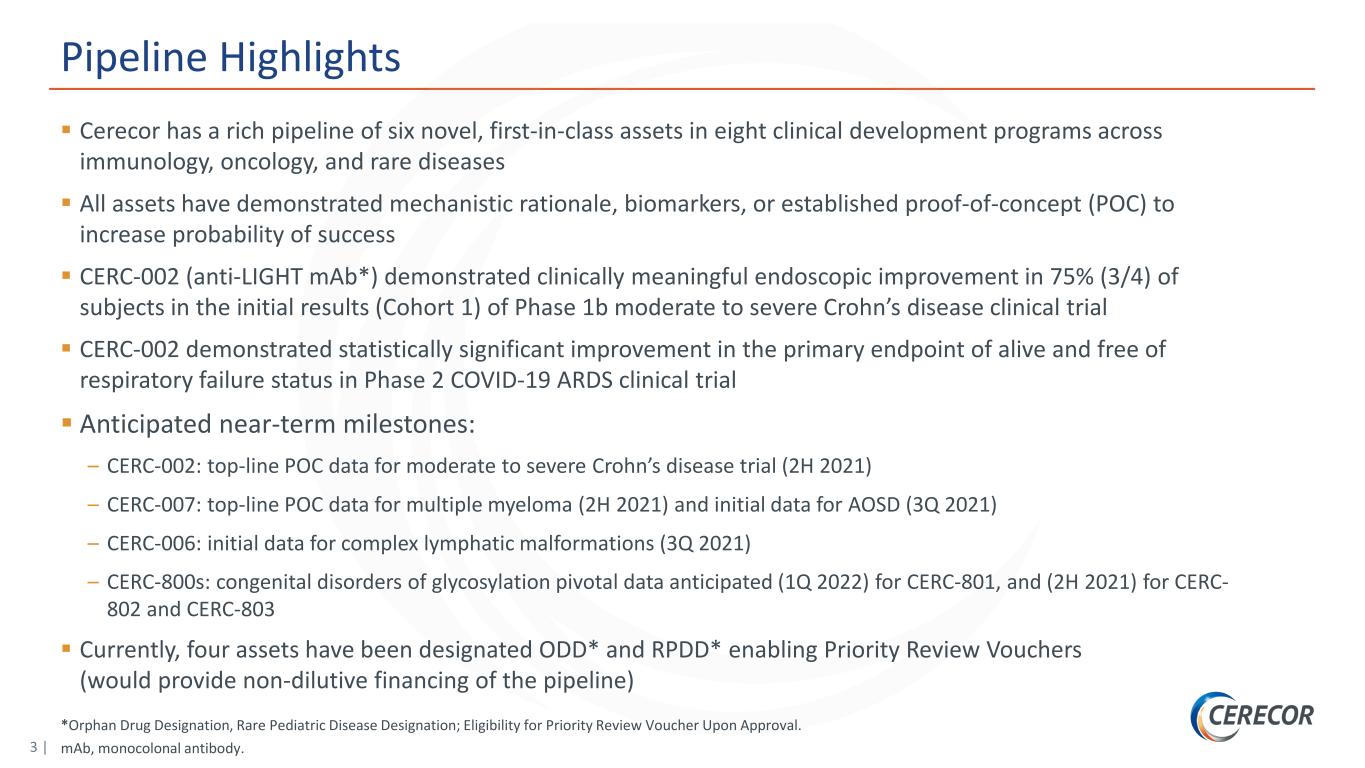
3 | *Orphan Drug Designation, Rare Pediatric Disease Designation; Eligibility for Priority Review Voucher Upon Approval. mAb, monocolonal antibody. Pipeline Highlights Cerecor has a rich pipeline of six novel, first-in-class assets in eight clinical development programs across immunology, oncology, and rare diseases All assets have demonstrated mechanistic rationale, biomarkers, or established proof-of-concept (POC) to increase probability of success CERC-002 (anti-LIGHT mAb*) demonstrated clinically meaningful endoscopic improvement in 75% (3/4) of subjects in the initial results (Cohort 1) of Phase 1b moderate to severe Crohn’s disease clinical trial CERC-002 demonstrated statistically significant improvement in the primary endpoint of alive and free of respiratory failure status in Phase 2 COVID-19 ARDS clinical trial Anticipated near-term milestones: – CERC-002: top-line POC data for moderate to severe Crohn’s disease trial (2H 2021) – CERC-007: top-line POC data for multiple myeloma (2H 2021) and initial data for AOSD (3Q 2021) – CERC-006: initial data for complex lymphatic malformations (3Q 2021) – CERC-800s: congenital disorders of glycosylation pivotal data anticipated (1Q 2022) for CERC-801, and (2H 2021) for CERC- 802 and CERC-803 Currently, four assets have been designated ODD* and RPDD* enabling Priority Review Vouchers (would provide non-dilutive financing of the pipeline)
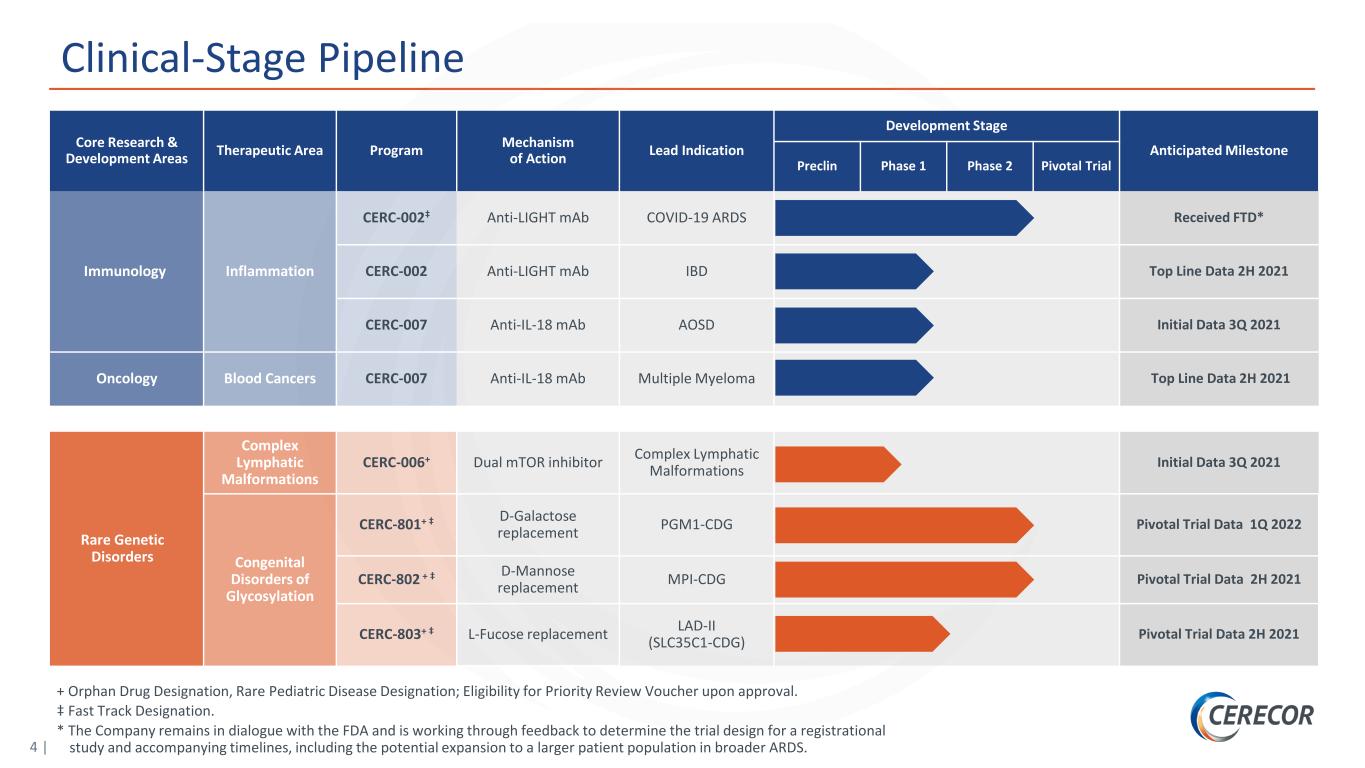
4 | Clinical-Stage Pipeline Core Research & Development Areas Therapeutic Area Program Mechanism of Action Lead Indication Development Stage Anticipated Milestone Preclin Phase 1 Phase 2 Pivotal Trial Immunology Inflammation CERC-002‡ Anti-LIGHT mAb COVID-19 ARDS Received FTD* CERC-002 Anti-LIGHT mAb IBD Top Line Data 2H 2021 CERC-007 Anti-IL-18 mAb AOSD Initial Data 3Q 2021 Oncology Blood Cancers CERC-007 Anti-IL-18 mAb Multiple Myeloma Top Line Data 2H 2021 Rare Genetic Disorders Complex Lymphatic Malformations CERC-006+ Dual mTOR inhibitor Complex Lymphatic Malformations Initial Data 3Q 2021 Congenital Disorders of Glycosylation CERC-801+ ‡ D-Galactose replacement PGM1-CDG Pivotal Trial Data 1Q 2022 CERC-802 + ‡ D-Mannose replacement MPI-CDG Pivotal Trial Data 2H 2021 CERC-803+ ‡ L-Fucose replacement LAD-II (SLC35C1-CDG) Pivotal Trial Data 2H 2021 + Orphan Drug Designation, Rare Pediatric Disease Designation; Eligibility for Priority Review Voucher upon approval. ‡ Fast Track Designation. * The Company remains in dialogue with the FDA and is working through feedback to determine the trial design for a registrational study and accompanying timelines, including the potential expansion to a larger patient population in broader ARDS.

Anti-LIGHT monoclonal antibody in clinical studies for Crohn’s disease and COVID-19 ARDS CERC-002
6 | CERC-002: A Novel First-in-Class Anti-LIGHT (TNFSF14) mAb In-licensed From Kyowa Kirin Co., Worldwide Exclusive Rights* for All Indications (2021) (2021) *Kyowa Kirin has an option to retain the rights in Japan. Novel, first-in-class fully human subcutaneous (SQ) monoclonal antibody (mAb) Only known fully human anti-LIGHT mAb Only known anti-LIGHT mAb in clinical development

CERC-002: Crohn’s Disease

8 | Executive Summary: CERC-002 Demonstrates Potential Proof-of-Concept in Initial Low-Dose Cohort Open-label proof-of-concept study in patients with moderate to severe Crohn’s disease who previously failed 3 or more lines of biologic therapies, including anti-TNFα* Clinically meaningful mucosal healing, determined by colonoscopy, in 3 of 4 subjects (SES-CD)** Rapid response within 8 weeks Well-tolerated, no serious adverse events observed High-dose cohort fully enrolled with results expected 2H21 *TNFα, tumor necrosis factor alpha; **SES-CD, Simple Endoscopic Score for Crohn’s Disease. 2nd Positive Proof-of-Concept Study With CERC-002 Further Validates the LIGHT MOA in Inflammatory Diseases
9 | 1. Centers for Disease Control and Prevention. Inflammatory bowel disease. https://www.cdc.gov/ibd/features/IBD-more-chronic-diseases.html. Accessed July 17, 2021. 2. Atreya R et al. Front Med (Lausanne). 2020;7:517. 3. Knowles SR et al. Inflamm Bowel Dis. 2018;24(4):742-751. 4. Byron C et al. J Clin Nurs. 2020;29(3-4):305-319. 5. GBD 2017 Inflammatory Bowel Disease Collaborators. Lancet. 2020;5:17-30. 6. Crohn’s and Colitis Foundation of America.The facts about inflammatory bowel disease. https://www.crohnscolitisfoundation.org/sites/default/files/2019- 02/Updated%20IBD%20Factbook.pdf. Accessed July 19, 2021. 7. Shivashankar R et al. Clin Gastroenterol Hepatol. 2017;15(6):857-863. 8. EMR Reports. https://www.expertmarketresearch.com/reports/inflammatory- bowel-disease-treatment-market. Accessed July 17, 2021. Inflammatory Bowel Disease Overview Inflammatory bowel disease (IBD) is a broad term indicating chronic inflammation of the gastrointestinal (GI) tract and includes both Crohn’s disease (CD) and ulcerative colitis (UC)1 – Relapsing and remitting course characterized by intestinal inflammation and epithelial injury, causing lifelong morbidity2 that significantly impacts quality of life3,4 Standard of care relies on treating the inflammatory activity that causes strictures, fistula, and abscesses as well as heightened incidence of colitis-associated neoplasia associated with IBD5 An estimated 1.6-3.1 million US adults (~1.3%) have a diagnosis of IBD1,6 – Estimated US cases of CD as many as 780K7 Approximately $16.7B global market opportunity in 2020 with 4.5% compound annual growth rate8 Significant Unmet Need Exists in Crohn’s Disease and Ulcerative Colitis
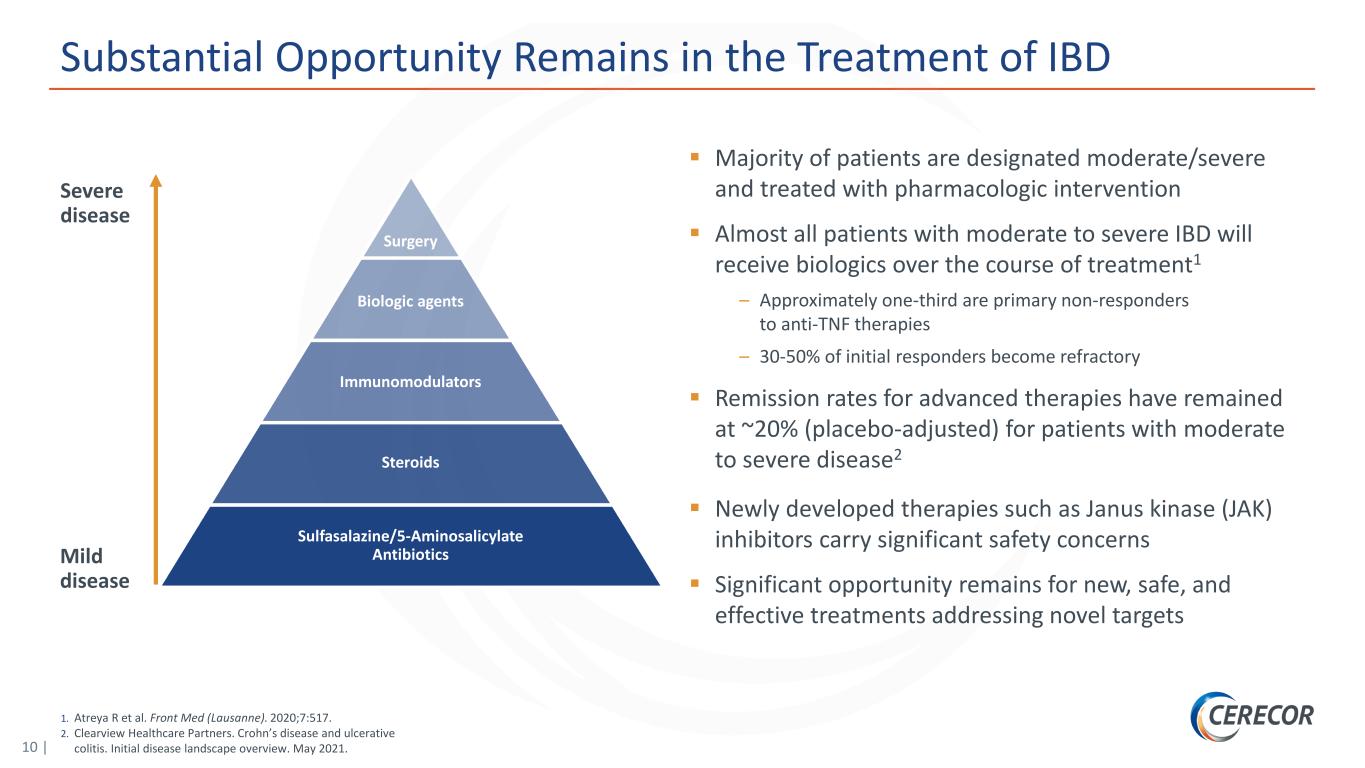
10 | 1. Atreya R et al. Front Med (Lausanne). 2020;7:517. 2. Clearview Healthcare Partners. Crohn’s disease and ulcerative colitis. Initial disease landscape overview. May 2021. Substantial Opportunity Remains in the Treatment of IBD Majority of patients are designated moderate/severe and treated with pharmacologic intervention Almost all patients with moderate to severe IBD will receive biologics over the course of treatment1 – Approximately one-third are primary non-responders to anti-TNF therapies – 30-50% of initial responders become refractory Remission rates for advanced therapies have remained at ~20% (placebo-adjusted) for patients with moderate to severe disease2 Newly developed therapies such as Janus kinase (JAK) inhibitors carry significant safety concerns Significant opportunity remains for new, safe, and effective treatments addressing novel targets Severe disease Mild disease Surgery Biologic agents Immunomodulators Steroids Sulfasalazine/5-Aminosalicylate Antibiotics
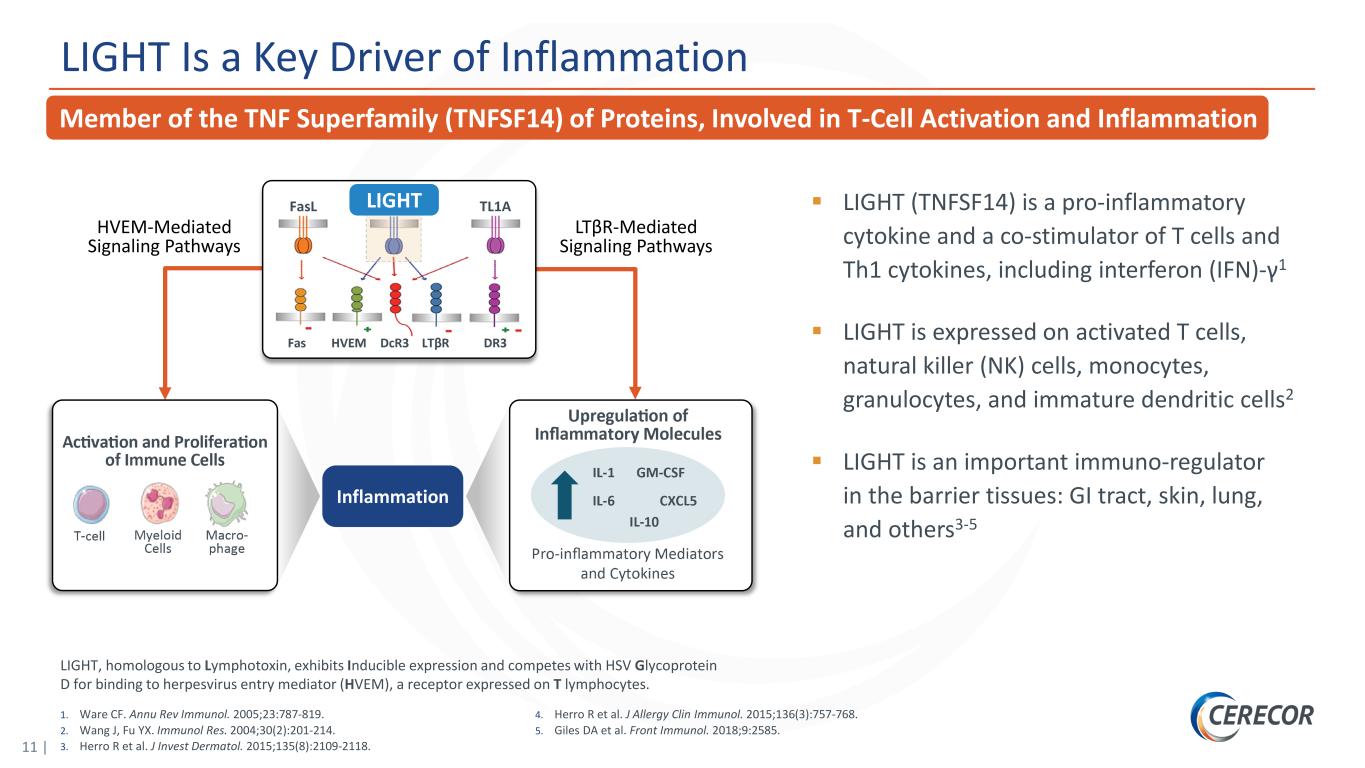
11 | LIGHT Is a Key Driver of Inflammation LIGHT Inflammation HVEM-Mediated Signaling Pathways LTβR-Mediated Signaling Pathways LIGHT (TNFSF14) is a pro-inflammatory cytokine and a co-stimulator of T cells and Th1 cytokines, including interferon (IFN)-γ1 LIGHT is expressed on activated T cells, natural killer (NK) cells, monocytes, granulocytes, and immature dendritic cells2 LIGHT is an important immuno-regulator in the barrier tissues: GI tract, skin, lung, and others3-5 1. Ware CF. Annu Rev Immunol. 2005;23:787-819. 2. Wang J, Fu YX. Immunol Res. 2004;30(2):201-214. 3. Herro R et al. J Invest Dermatol. 2015;135(8):2109-2118. 4. Herro R et al. J Allergy Clin Immunol. 2015;136(3):757-768. 5. Giles DA et al. Front Immunol. 2018;9:2585. Member of the TNF Superfamily (TNFSF14) of Proteins, Involved in T-Cell Activation and Inflammation LIGHT, homologous to Lymphotoxin, exhibits Inducible expression and competes with HSV Glycoprotein D for binding to herpesvirus entry mediator (HVEM), a receptor expressed on T lymphocytes.
12 | 1. Data on file, Cerecor, Inc. 2. Moraes L et al. Inflamm Bowel Dis. 2020;26(6):874-884. 3. Cohavy O et al. J Immunol. 2005;174(2):646-653. 4. Wang J et al. J Immunol. 2005;174(12):8173-8182. 5. Shaikh RB et al. J Immunol. 2001;167(11):6330-6337. 6. Jungbeck M et al. Immunology. 2009;128(3):451-458. 7. Schaer C et al. PLoS One. 2011;6(4):e18495. Multiple Lines of Evidence Support Importance of LIGHT in IBD Patient data ‒ Elevated levels of LIGHT in patients with CD1 and UC2 ‒ High LIGHT mRNA levels detected in human inflamed intestinal tissue compared with normal tissue1,3 ‒ LIGHT gene upregulation is observed in CD and UC4 Animal models of IBD ‒ LIGHT overexpression increases intestinal inflammation in rodents5 ‒ Anti-LIGHT monoclonal antibody (mAb) treatment ameliorates inflammation in the dextrate sulfate sodium (DSS)–induced colitis model6 ‒ Knockout of the LIGHT (or its ligand, HVEM) gene results in reduced intestinal inflammation (in some models)7
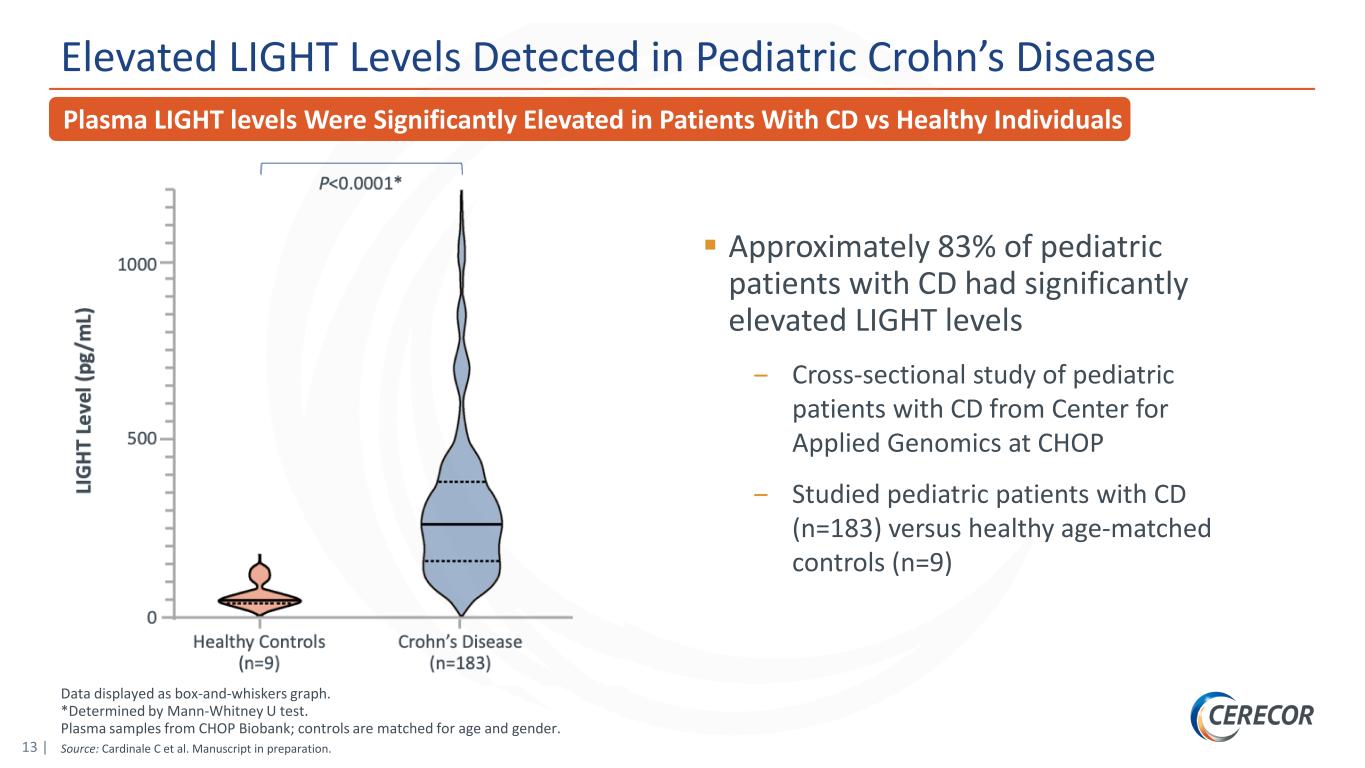
13 | Data displayed as box-and-whiskers graph. *Determined by Mann-Whitney U test. Plasma samples from CHOP Biobank; controls are matched for age and gender. Source: Cardinale C et al. Manuscript in preparation. Elevated LIGHT Levels Detected in Pediatric Crohn’s Disease Approximately 83% of pediatric patients with CD had significantly elevated LIGHT levels ‒ Cross-sectional study of pediatric patients with CD from Center for Applied Genomics at CHOP ‒ Studied pediatric patients with CD (n=183) versus healthy age-matched controls (n=9) Plasma LIGHT levels Were Significantly Elevated in Patients With CD vs Healthy Individuals
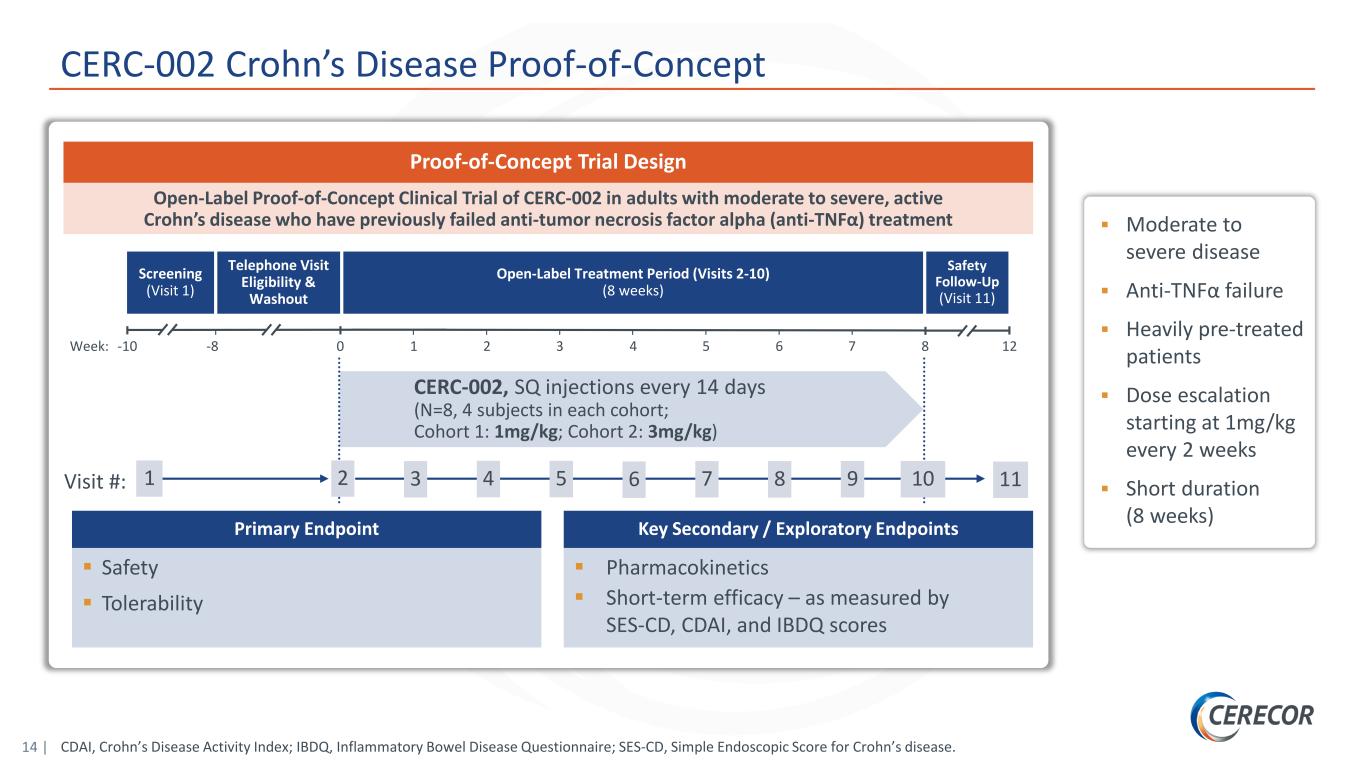
14 | Screening (Visit 1) Open-Label Treatment Period (Visits 2-10) (8 weeks) Safety Follow-Up (Visit 11) CERC-002, SQ injections every 14 days (N=8, 4 subjects in each cohort; Cohort 1: 1mg/kg; Cohort 2: 3mg/kg) Telephone Visit Eligibility & Washout Week: -10 0 1 2 3 4 5 6-8 127 8 Visit #: 1 2 3 4 5 6 7 108 9 11 Primary Endpoint Safety Tolerability Key Secondary / Exploratory Endpoints Pharmacokinetics Short-term efficacy – as measured by SES-CD, CDAI, and IBDQ scores Open-Label Proof-of-Concept Clinical Trial of CERC-002 in adults with moderate to severe, active Crohn’s disease who have previously failed anti-tumor necrosis factor alpha (anti-TNFα) treatment Proof-of-Concept Trial Design Moderate to severe disease Anti-TNFα failure Heavily pre-treated patients Dose escalation starting at 1mg/kg every 2 weeks Short duration (8 weeks) CERC-002 Crohn’s Disease Proof-of-Concept CDAI, Crohn’s Disease Activity Index; IBDQ, Inflammatory Bowel Disease Questionnaire; SES-CD, Simple Endoscopic Score for Crohn’s disease.
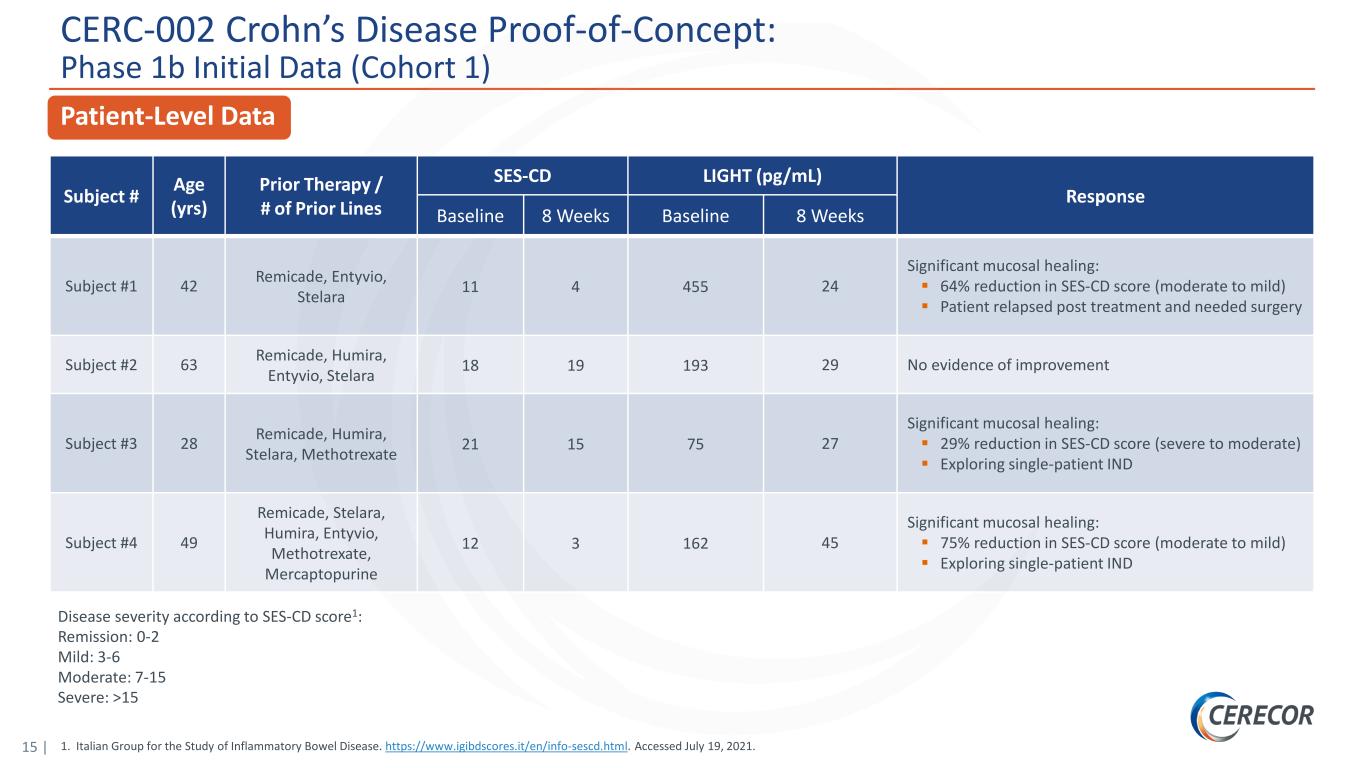
15 | CERC-002 Crohn’s Disease Proof-of-Concept: Phase 1b Initial Data (Cohort 1) Patient-Level Data Disease severity according to SES-CD score1: Remission: 0-2 Mild: 3-6 Moderate: 7-15 Severe: >15 Subject # Age (yrs) Prior Therapy / # of Prior Lines SES-CD LIGHT (pg/mL) Response Baseline 8 Weeks Baseline 8 Weeks Subject #1 42 Remicade, Entyvio, Stelara 11 4 455 24 Significant mucosal healing: 64% reduction in SES-CD score (moderate to mild) Patient relapsed post treatment and needed surgery Subject #2 63 Remicade, Humira, Entyvio, Stelara 18 19 193 29 No evidence of improvement Subject #3 28 Remicade, Humira, Stelara, Methotrexate 21 15 75 27 Significant mucosal healing: 29% reduction in SES-CD score (severe to moderate) Exploring single-patient IND Subject #4 49 Remicade, Stelara, Humira, Entyvio, Methotrexate, Mercaptopurine 12 3 162 45 Significant mucosal healing: 75% reduction in SES-CD score (moderate to mild) Exploring single-patient IND 1. Italian Group for the Study of Inflammatory Bowel Disease. https://www.igibdscores.it/en/info-sescd.html. Accessed July 19, 2021.
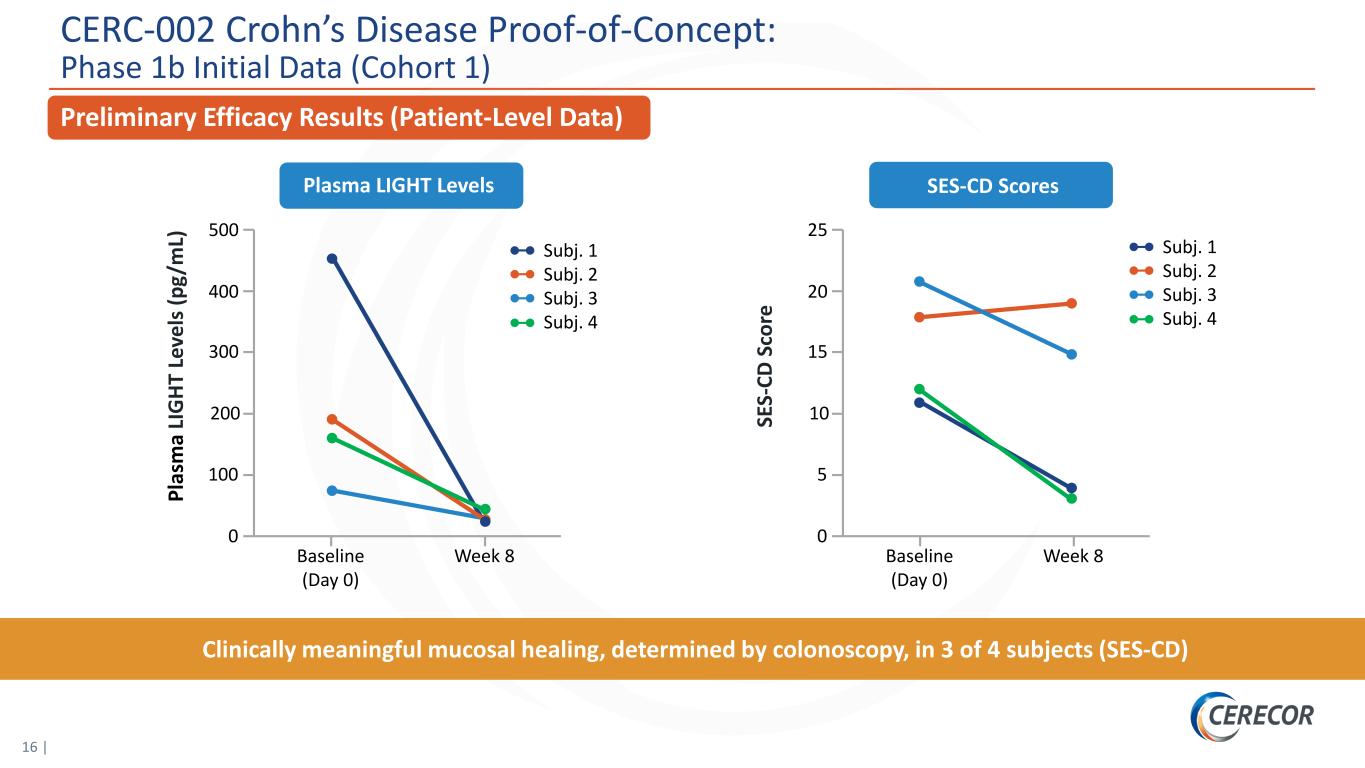
16 | Preliminary Efficacy Results (Patient-Level Data) SES-CD ScoresPlasma LIGHT Levels Clinically meaningful mucosal healing, determined by colonoscopy, in 3 of 4 subjects (SES-CD) SE S- CD S co re 25 Baseline (Day 0) Week 8 20 10 5 0 15 Pl as m a LI G HT L ev el s ( pg /m L) 500 Baseline (Day 0) Week 8 400 200 100 0 300 Subj. 1 Subj. 2 Subj. 3 Subj. 4 Subj. 1 Subj. 2 Subj. 3 Subj. 4 CERC-002 Crohn’s Disease Proof-of-Concept: Phase 1b Initial Data (Cohort 1)
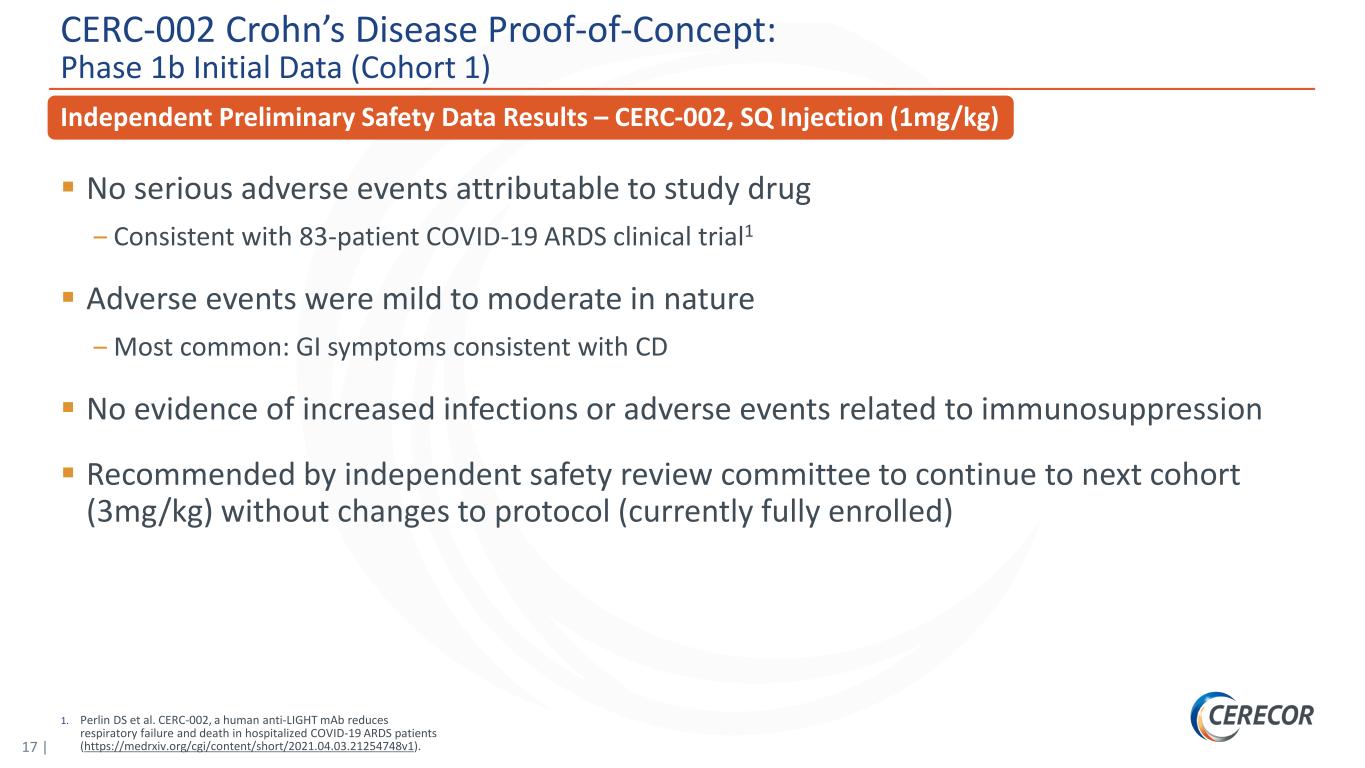
17 | 1. Perlin DS et al. CERC-002, a human anti-LIGHT mAb reduces respiratory failure and death in hospitalized COVID-19 ARDS patients (https://medrxiv.org/cgi/content/short/2021.04.03.21254748v1). Independent Preliminary Safety Data Results – CERC-002, SQ Injection (1mg/kg) No serious adverse events attributable to study drug ‒ Consistent with 83-patient COVID-19 ARDS clinical trial1 Adverse events were mild to moderate in nature ‒ Most common: GI symptoms consistent with CD No evidence of increased infections or adverse events related to immunosuppression Recommended by independent safety review committee to continue to next cohort (3mg/kg) without changes to protocol (currently fully enrolled) CERC-002 Crohn’s Disease Proof-of-Concept: Phase 1b Initial Data (Cohort 1)

CERC-002: COVID-19 ARDS
19 | Phase 2 Clinical Trial Met Primary Endpoint in Patients Hospitalized with COVID-19 ARDS Executive Summary: Final Data Analysis CERC-002 significantly reduced respiratory failure and mortality in Phase 2 clinical trial in patients hospitalized with COVID-19 acute respiratory distress syndrome (ARDS) – This analysis updates the preliminary top-line data reported on January 5, 2021, and is inclusive of 60-day safety data – Hospitalized COVID-19 patients treated with a single dose of CERC-002 demonstrated statistically significant improvement in the primary endpoint (proportion of patients alive and free of respiratory failure over the 28-day study period) compared with placebo (n=62, P=0.044) – Efficacy was highest in a prespecified subpopulation of patients aged ≥60 years (n=34, P=0.042), the population most vulnerable to severe complications and death with COVID-19 infection – At both the 28-day and the 60-day final timepoints, an approximately 50% trend in mortality reduction (22.5% vs 10.8%) was observed – CERC-002 showed statistically significant efficacy on top of corticosteroids and standard of care in COVID-19 ARDS (~88% of patients in the trial received corticosteroids and ~58% received remdesivir) CERC-002 was well tolerated, with no appreciable differences in immunosuppression or other serious adverse events between CERC-002 and placebo CERC-002 dramatically and rapidly reduced serum free LIGHT levels – ~85% reduction in free LIGHT achieved in 1 day CERC-002 recently granted Fast Track designation for the treatment of hospitalized patients with COVID-19 Additionally, the company is exploring the applicability of CERC-002 in non-COVID-19 ARDS Source: Data on file, Cerecor Inc.
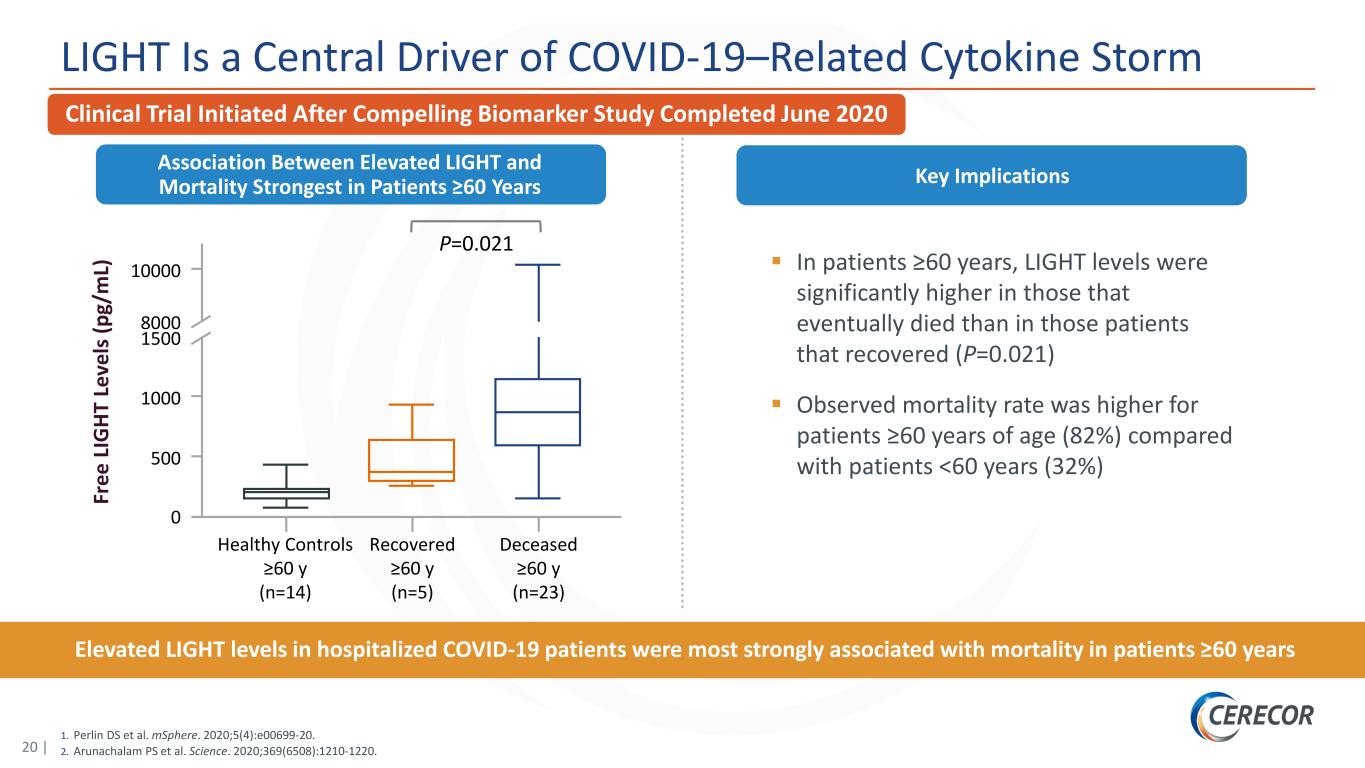
20 | 1. Perlin DS et al. mSphere. 2020;5(4):e00699-20. 2. Arunachalam PS et al. Science. 2020;369(6508):1210-1220. LIGHT Is a Central Driver of COVID-19–Related Cytokine Storm Clinical Trial Initiated After Compelling Biomarker Study Completed June 2020 In patients ≥60 years, LIGHT levels were significantly higher in those that eventually died than in those patients that recovered (P=0.021) Observed mortality rate was higher for patients ≥60 years of age (82%) compared with patients <60 years (32%) Key Implications Elevated LIGHT levels in hospitalized COVID-19 patients were most strongly associated with mortality in patients ≥60 years Association Between Elevated LIGHT and Mortality Strongest in Patients ≥60 Years Fr ee L IG HT L ev el s ( pg /m L) 10000 8000 1500 1000 500 0 Healthy Controls ≥60 y (n=14) Recovered ≥60 y (n=5) Deceased ≥60 y (n=23) P=0.021
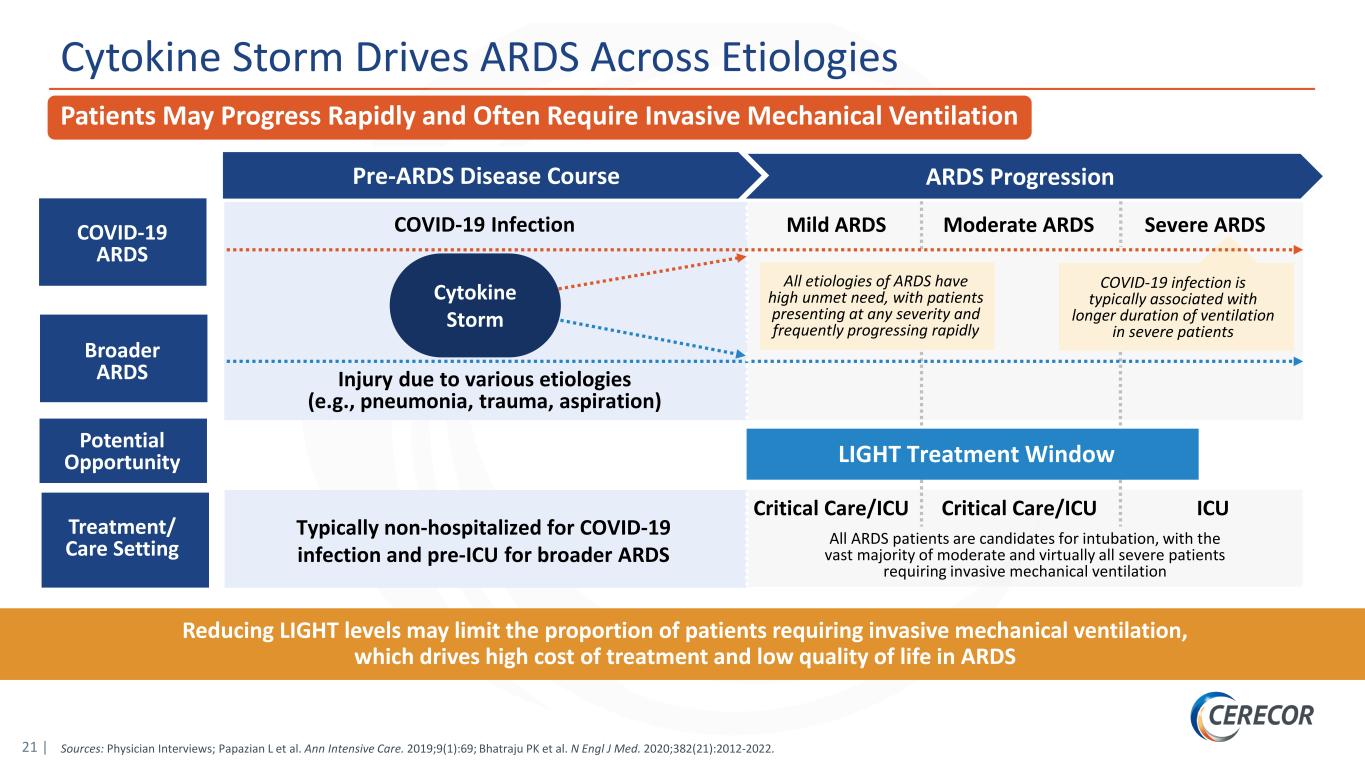
21 | Cytokine Storm Drives ARDS Across Etiologies Reducing LIGHT levels may limit the proportion of patients requiring invasive mechanical ventilation, which drives high cost of treatment and low quality of life in ARDS ARDS ProgressionPre-ARDS Disease Course All ARDS patients are candidates for intubation, with the vast majority of moderate and virtually all severe patients requiring invasive mechanical ventilation Critical Care/ICU ICUCritical Care/ICU COVID-19 Infection Injury due to various etiologies (e.g., pneumonia, trauma, aspiration) Typically non-hospitalized for COVID-19 infection and pre-ICU for broader ARDS COVID-19 ARDS Mild ARDS Moderate ARDS Severe ARDS Broader ARDS Potential Opportunity Treatment/ Care Setting All etiologies of ARDS have high unmet need, with patients presenting at any severity and frequently progressing rapidly COVID-19 infection is typically associated with longer duration of ventilation in severe patients LIGHT Treatment Window Patients May Progress Rapidly and Often Require Invasive Mechanical Ventilation Sources: Physician Interviews; Papazian L et al. Ann Intensive Care. 2019;9(1):69; Bhatraju PK et al. N Engl J Med. 2020;382(21):2012-2022. Cytokine Storm
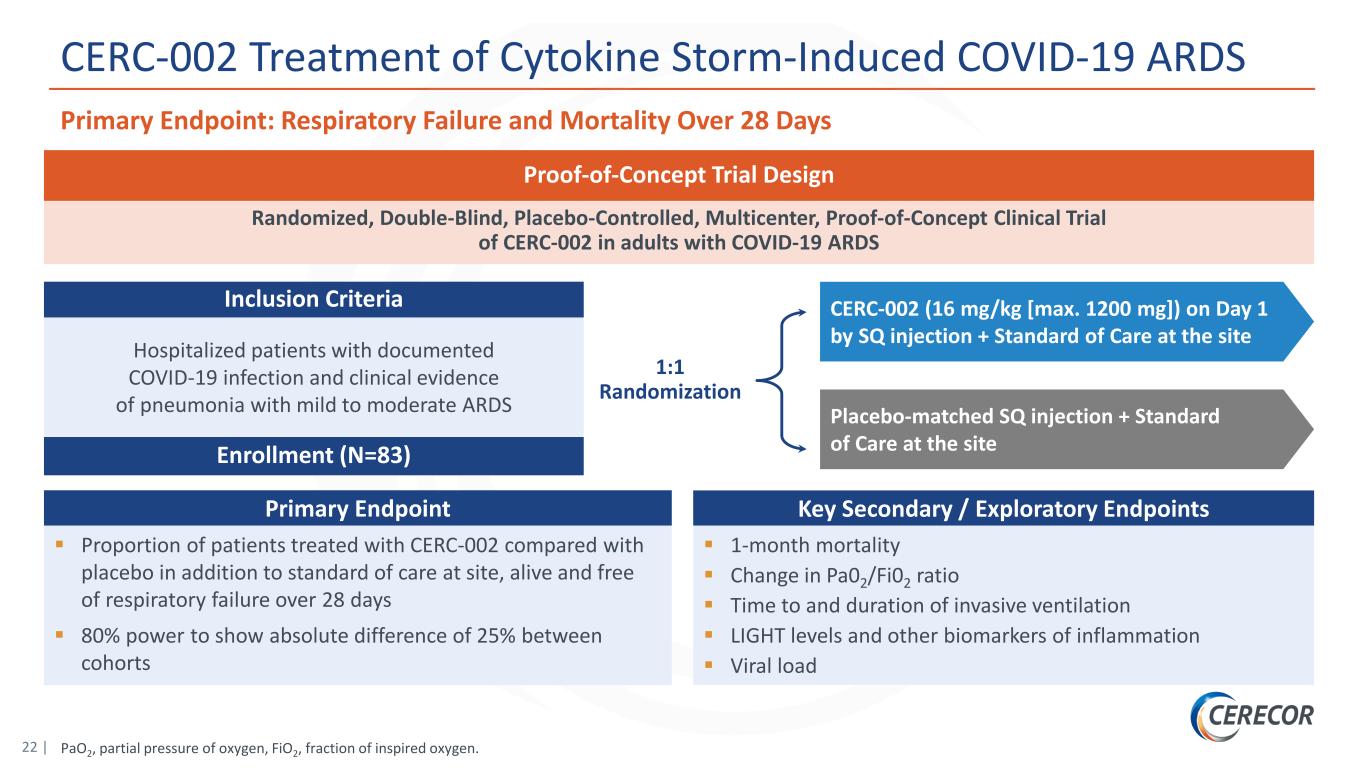
22 | PaO2, partial pressure of oxygen, FiO2, fraction of inspired oxygen. CERC-002 Treatment of Cytokine Storm-Induced COVID-19 ARDS 1:1 Randomization CERC-002 (16 mg/kg [max. 1200 mg]) on Day 1 by SQ injection + Standard of Care at the site Placebo-matched SQ injection + Standard of Care at the site Randomized, Double-Blind, Placebo-Controlled, Multicenter, Proof-of-Concept Clinical Trial of CERC-002 in adults with COVID-19 ARDS Hospitalized patients with documented COVID-19 infection and clinical evidence of pneumonia with mild to moderate ARDS Enrollment (N=83) Inclusion Criteria Proof-of-Concept Trial Design Primary Endpoint Proportion of patients treated with CERC-002 compared with placebo in addition to standard of care at site, alive and free of respiratory failure over 28 days 80% power to show absolute difference of 25% between cohorts Key Secondary / Exploratory Endpoints 1-month mortality Change in Pa02/Fi02 ratio Time to and duration of invasive ventilation LIGHT levels and other biomarkers of inflammation Viral load Primary Endpoint: Respiratory Failure and Mortality Over 28 Days
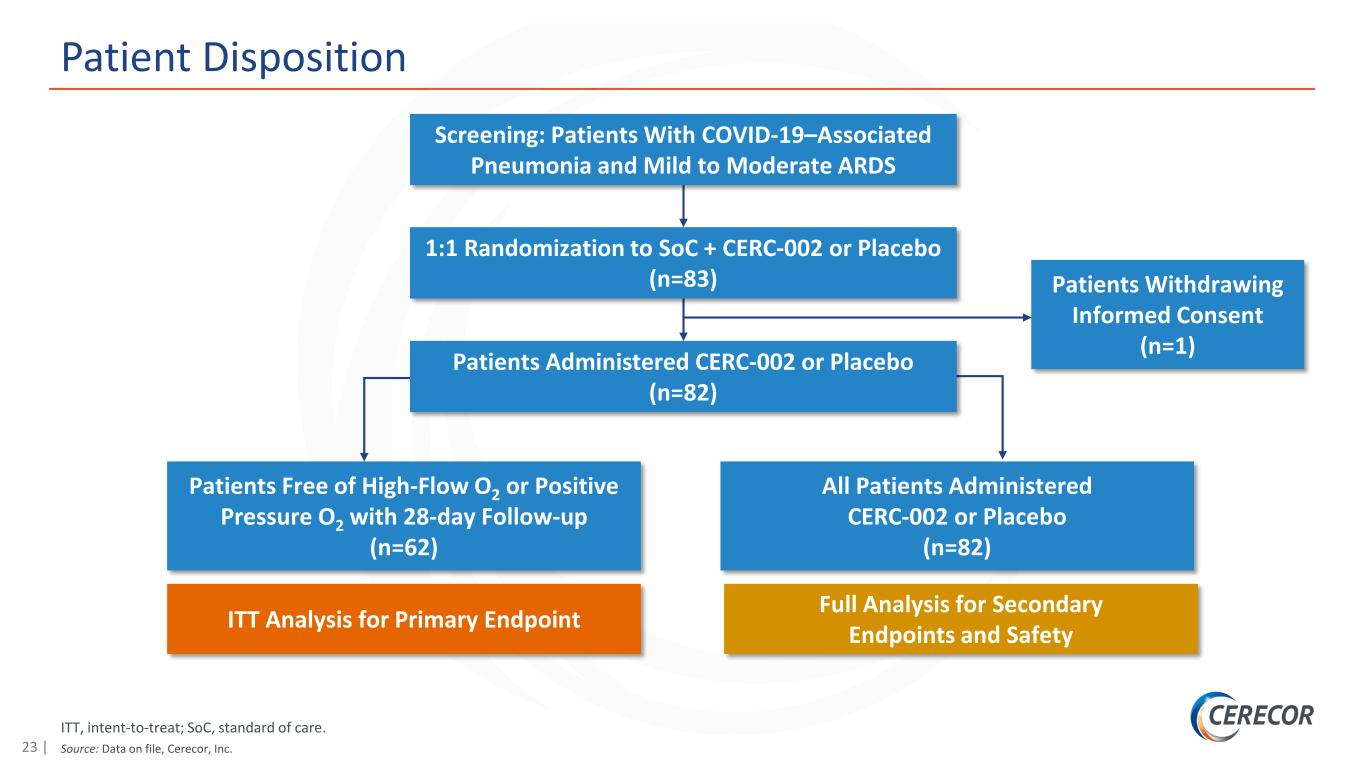
23 | ITT, intent-to-treat; SoC, standard of care. Source: Data on file, Cerecor, Inc. Patient Disposition Screening: Patients With COVID-19–Associated Pneumonia and Mild to Moderate ARDS 1:1 Randomization to SoC + CERC-002 or Placebo (n=83) Patients Administered CERC-002 or Placebo (n=82) Patients Withdrawing Informed Consent (n=1) Patients Free of High-Flow O2 or Positive Pressure O2 with 28-day Follow-up (n=62) All Patients Administered CERC-002 or Placebo (n=82) ITT Analysis for Primary Endpoint Full Analysis for Secondary Endpoints and Safety
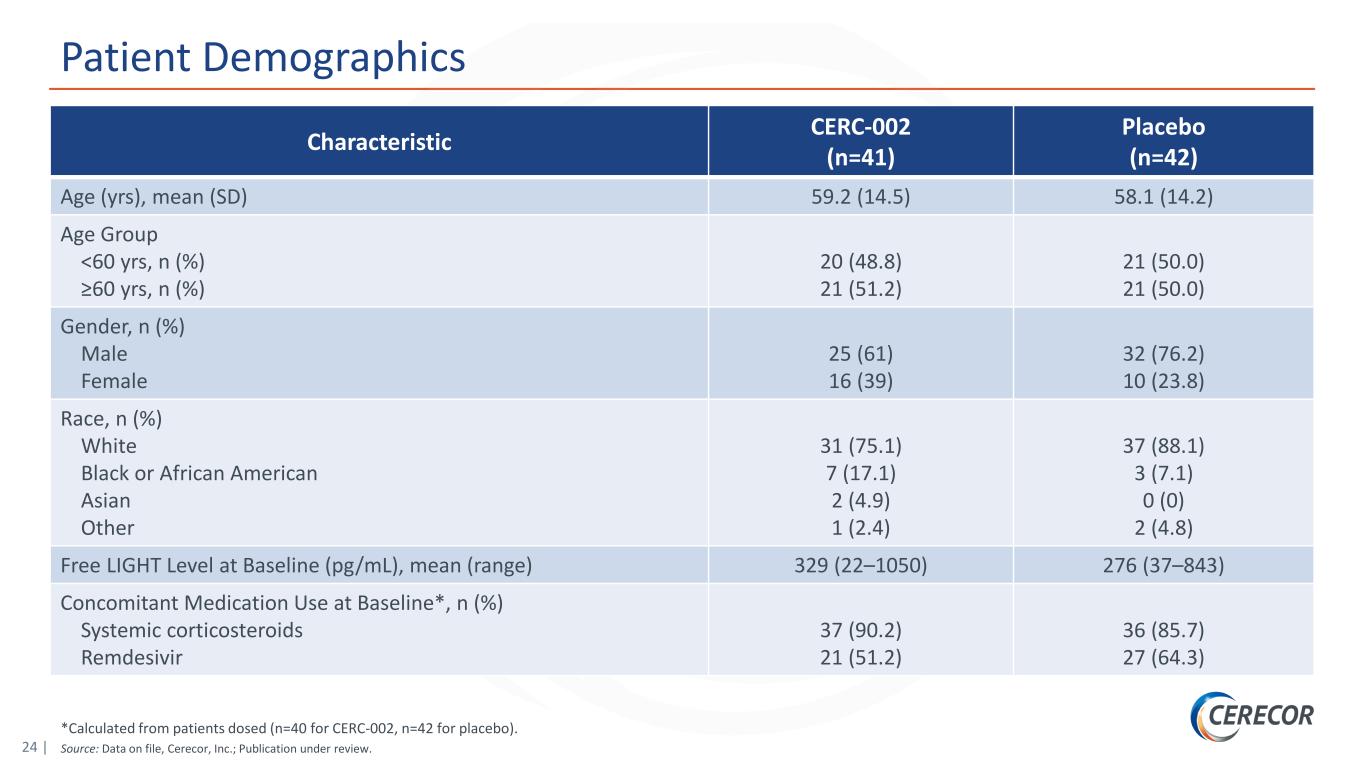
24 | *Calculated from patients dosed (n=40 for CERC-002, n=42 for placebo). Source: Data on file, Cerecor, Inc.; Publication under review. Patient Demographics Characteristic CERC-002 (n=41) Placebo (n=42) Age (yrs), mean (SD) 59.2 (14.5) 58.1 (14.2) Age Group <60 yrs, n (%) ≥60 yrs, n (%) 20 (48.8) 21 (51.2) 21 (50.0) 21 (50.0) Gender, n (%) Male Female 25 (61) 16 (39) 32 (76.2) 10 (23.8) Race, n (%) White Black or African American Asian Other 31 (75.1) 7 (17.1) 2 (4.9) 1 (2.4) 37 (88.1) 3 (7.1) 0 (0) 2 (4.8) Free LIGHT Level at Baseline (pg/mL), mean (range) 329 (22–1050) 276 (37–843) Concomitant Medication Use at Baseline*, n (%) Systemic corticosteroids Remdesivir 37 (90.2) 21 (51.2) 36 (85.7) 27 (64.3)
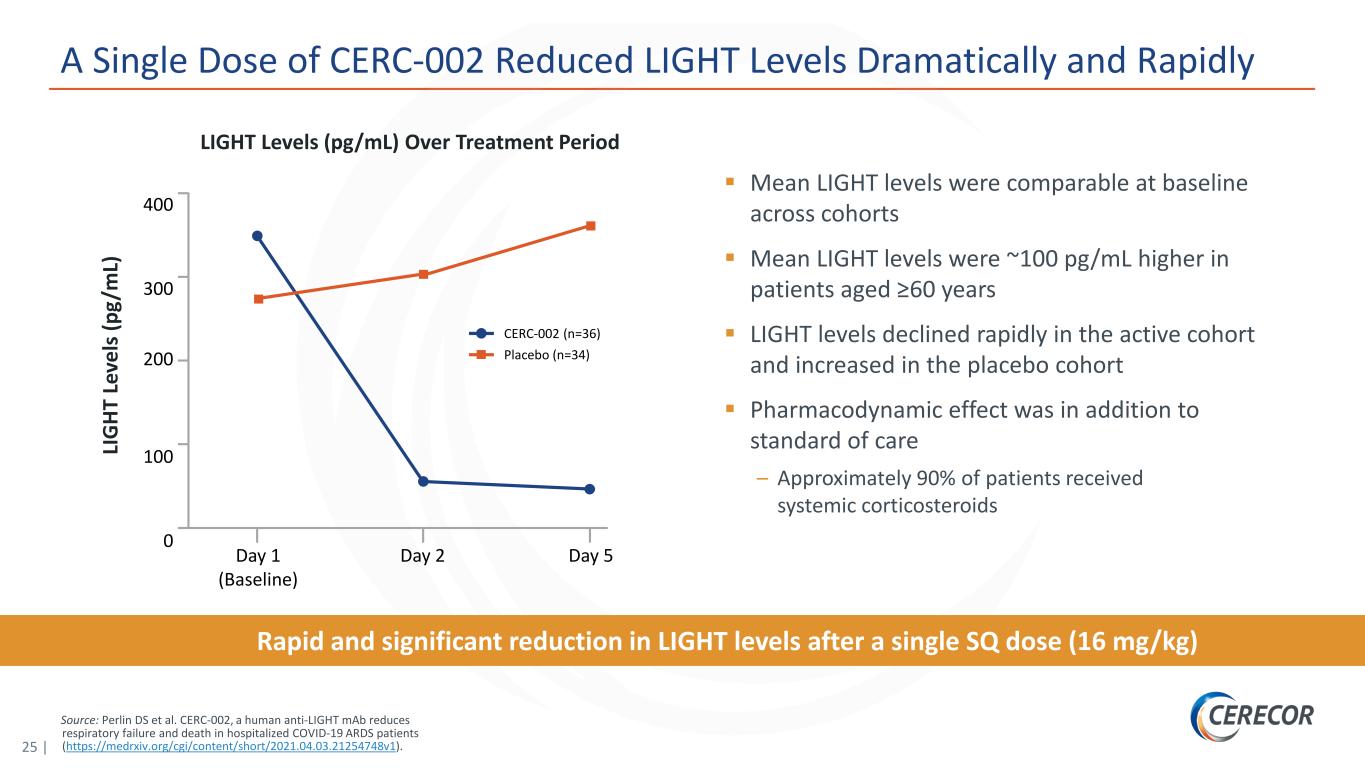
25 | Source: Perlin DS et al. CERC-002, a human anti-LIGHT mAb reduces respiratory failure and death in hospitalized COVID-19 ARDS patients (https://medrxiv.org/cgi/content/short/2021.04.03.21254748v1). A Single Dose of CERC-002 Reduced LIGHT Levels Dramatically and Rapidly Rapid and significant reduction in LIGHT levels after a single SQ dose (16 mg/kg) Mean LIGHT levels were comparable at baseline across cohorts Mean LIGHT levels were ~100 pg/mL higher in patients aged ≥60 years LIGHT levels declined rapidly in the active cohort and increased in the placebo cohort Pharmacodynamic effect was in addition to standard of care – Approximately 90% of patients received systemic corticosteroids LIGHT Levels (pg/mL) Over Treatment Period 400 300 100 0 CERC-002 (n=36) Placebo (n=34) LI G HT L ev el s ( pg /m L) 200 Day 5Day 1 (Baseline) Day 2
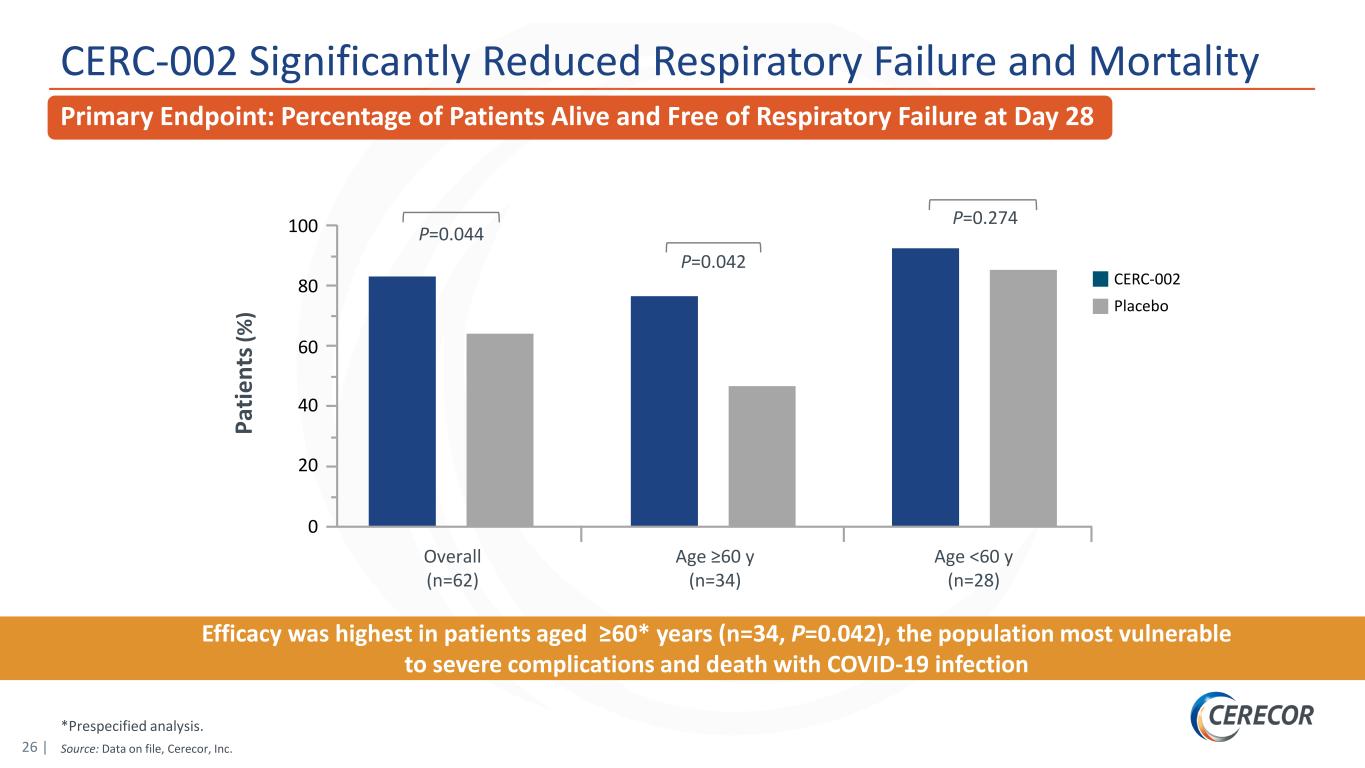
26 | *Prespecified analysis. Source: Data on file, Cerecor, Inc. CERC-002 Significantly Reduced Respiratory Failure and Mortality Efficacy was highest in patients aged ≥60* years (n=34, P=0.042), the population most vulnerable to severe complications and death with COVID-19 infection Pa tie nt s( % ) 100 60 40 20 0 CERC-002 Overall (n=62) Placebo Age ≥60 y (n=34) Age <60 y (n=28) 80 P=0.044 P=0.042 P=0.274 Primary Endpoint: Percentage of Patients Alive and Free of Respiratory Failure at Day 28
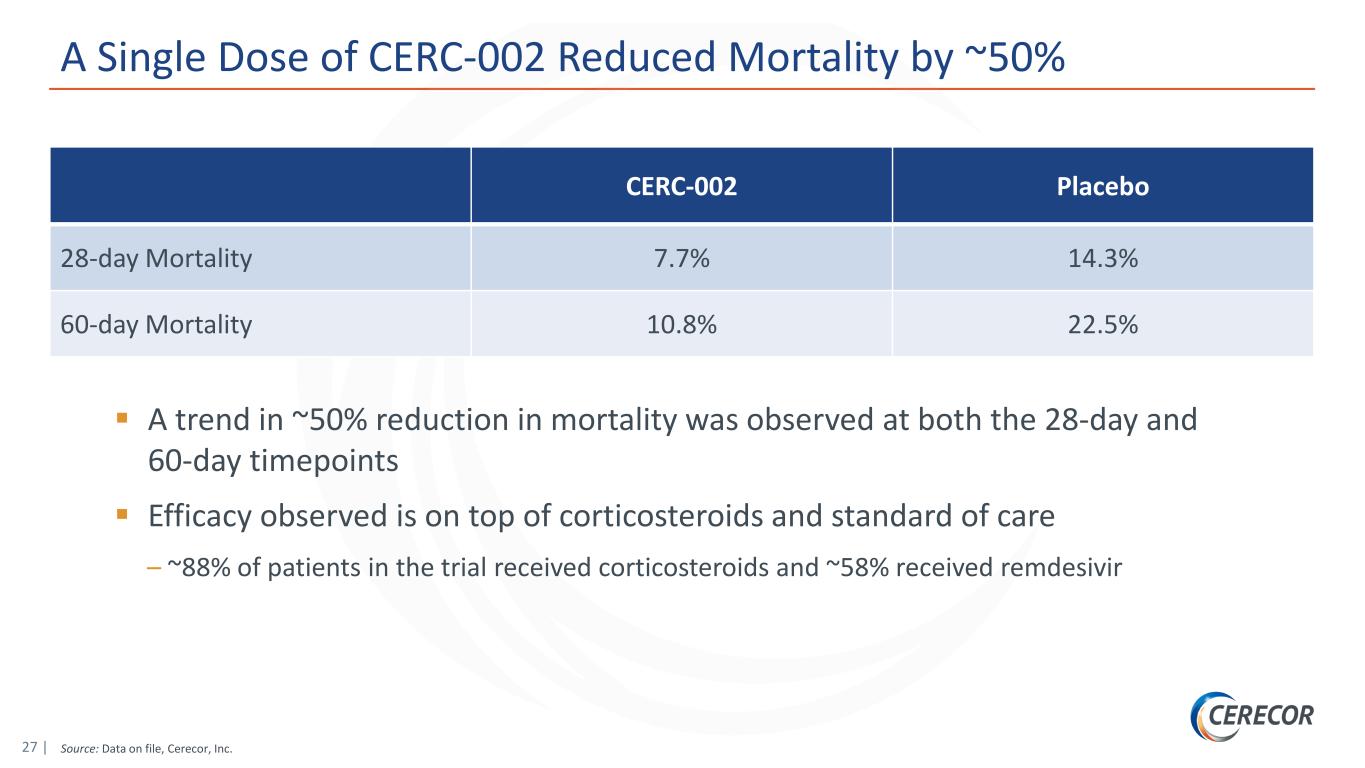
27 | Source: Data on file, Cerecor, Inc. A Single Dose of CERC-002 Reduced Mortality by ~50% CERC-002 Placebo 28-day Mortality 7.7% 14.3% 60-day Mortality 10.8% 22.5% A trend in ~50% reduction in mortality was observed at both the 28-day and 60-day timepoints Efficacy observed is on top of corticosteroids and standard of care – ~88% of patients in the trial received corticosteroids and ~58% received remdesivir
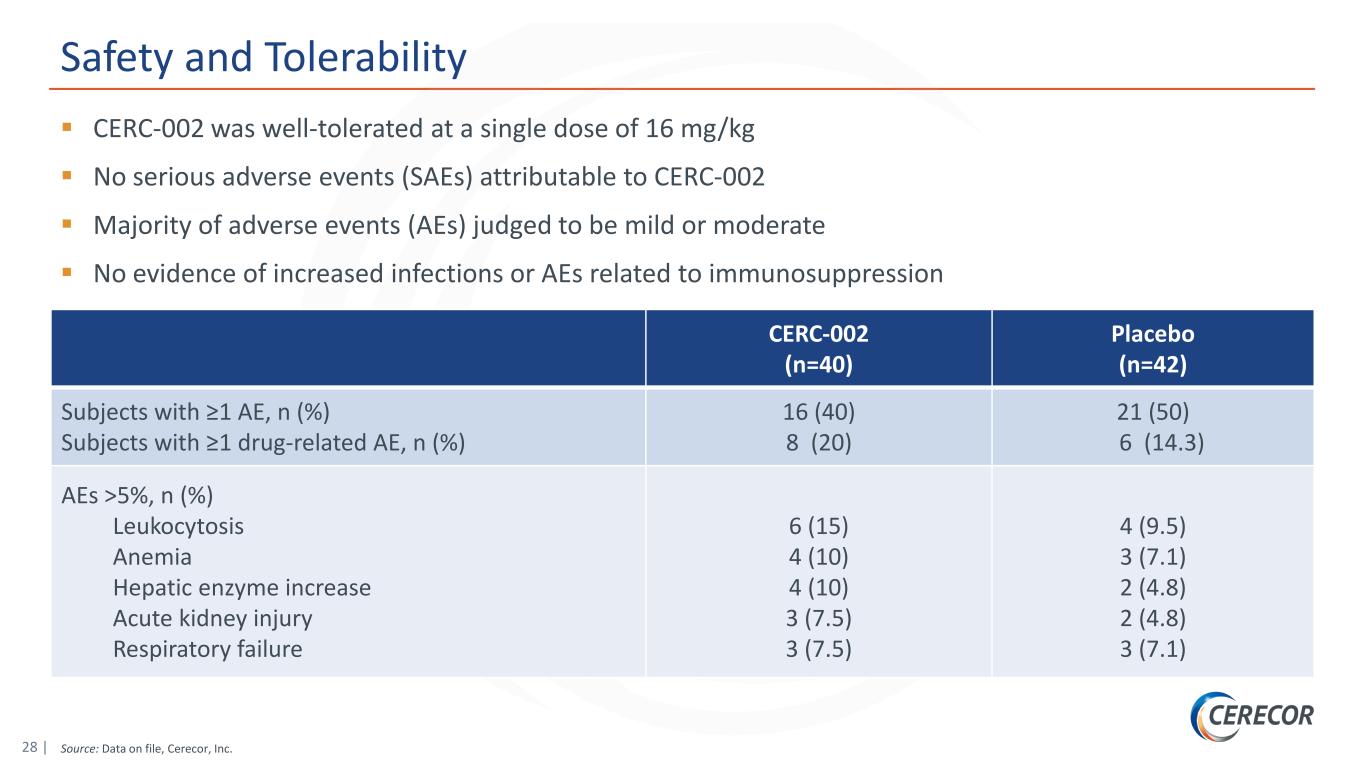
28 | Source: Data on file, Cerecor, Inc. Safety and Tolerability CERC-002 was well-tolerated at a single dose of 16 mg/kg No serious adverse events (SAEs) attributable to CERC-002 Majority of adverse events (AEs) judged to be mild or moderate No evidence of increased infections or AEs related to immunosuppression CERC-002 (n=40) Placebo (n=42) Subjects with ≥1 AE, n (%) Subjects with ≥1 drug-related AE, n (%) 16 (40) 8 (20) 21 (50) 6 (14.3) AEs >5%, n (%) Leukocytosis Anemia Hepatic enzyme increase Acute kidney injury Respiratory failure 6 (15) 4 (10) 4 (10) 3 (7.5) 3 (7.5) 4 (9.5) 3 (7.1) 2 (4.8) 2 (4.8) 3 (7.1)
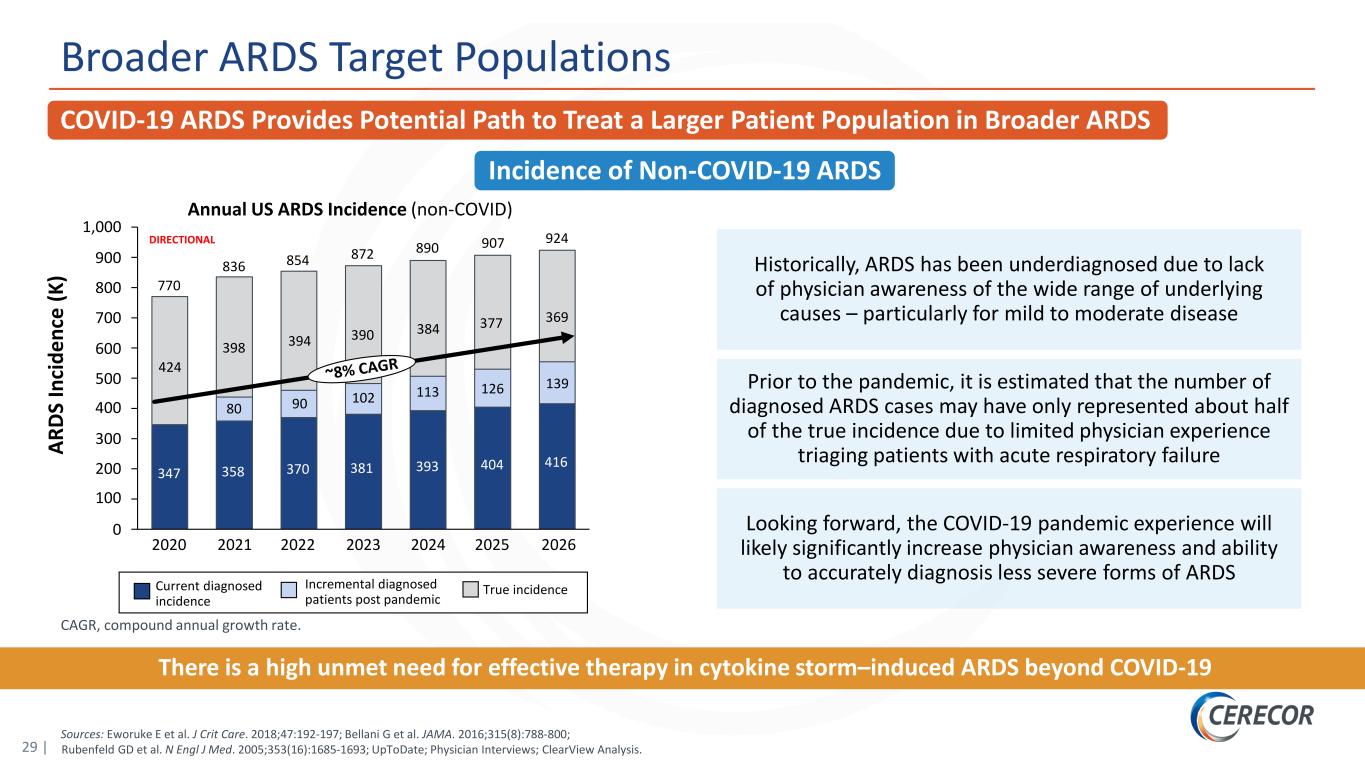
29 | CAGR, compound annual growth rate. Sources: Eworuke E et al. J Crit Care. 2018;47:192-197; Bellani G et al. JAMA. 2016;315(8):788-800; Rubenfeld GD et al. N Engl J Med. 2005;353(16):1685-1693; UpToDate; Physician Interviews; ClearView Analysis. COVID-19 ARDS Provides Potential Path to Treat a Larger Patient Population in Broader ARDS Broader ARDS Target Populations Incidence of Non-COVID-19 ARDS Annual US ARDS Incidence (non-COVID) AR DS In ci de nc e (K ) Historically, ARDS has been underdiagnosed due to lack of physician awareness of the wide range of underlying causes – particularly for mild to moderate disease Prior to the pandemic, it is estimated that the number of diagnosed ARDS cases may have only represented about half of the true incidence due to limited physician experience triaging patients with acute respiratory failure Looking forward, the COVID-19 pandemic experience will likely significantly increase physician awareness and ability to accurately diagnosis less severe forms of ARDS 400 200 800 300 500 0 100 600 1,000 700 900 90 2023 381 854 424 924 2026 347 398 2020 80 358 2021 394 370 770 393 2022 390 102 384 113 2024 416 139126 2025 369377 872 404 890 907 836 Current diagnosed incidence Incremental diagnosed patients post pandemic True incidence DIRECTIONAL There is a high unmet need for effective therapy in cytokine storm–induced ARDS beyond COVID-19
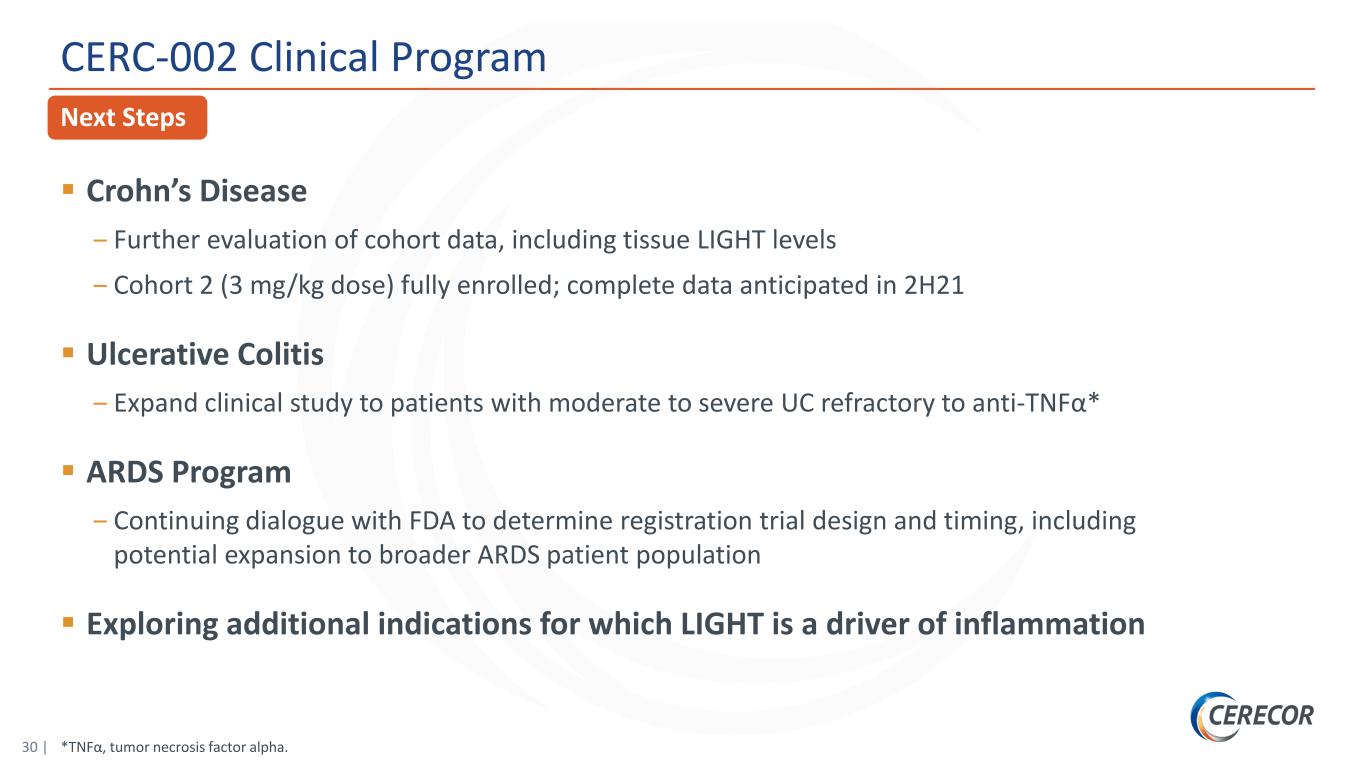
30 | *TNFα, tumor necrosis factor alpha. Next Steps CERC-002 Clinical Program Crohn’s Disease ‒ Further evaluation of cohort data, including tissue LIGHT levels ‒ Cohort 2 (3 mg/kg dose) fully enrolled; complete data anticipated in 2H21 Ulcerative Colitis ‒ Expand clinical study to patients with moderate to severe UC refractory to anti-TNFα* ARDS Program ‒ Continuing dialogue with FDA to determine registration trial design and timing, including potential expansion to broader ARDS patient population Exploring additional indications for which LIGHT is a driver of inflammation

Phase 1b anti-IL-18 monoclonal antibody for Multiple Myeloma and Still’s Disease (AOSD and sJIA) CERC-007
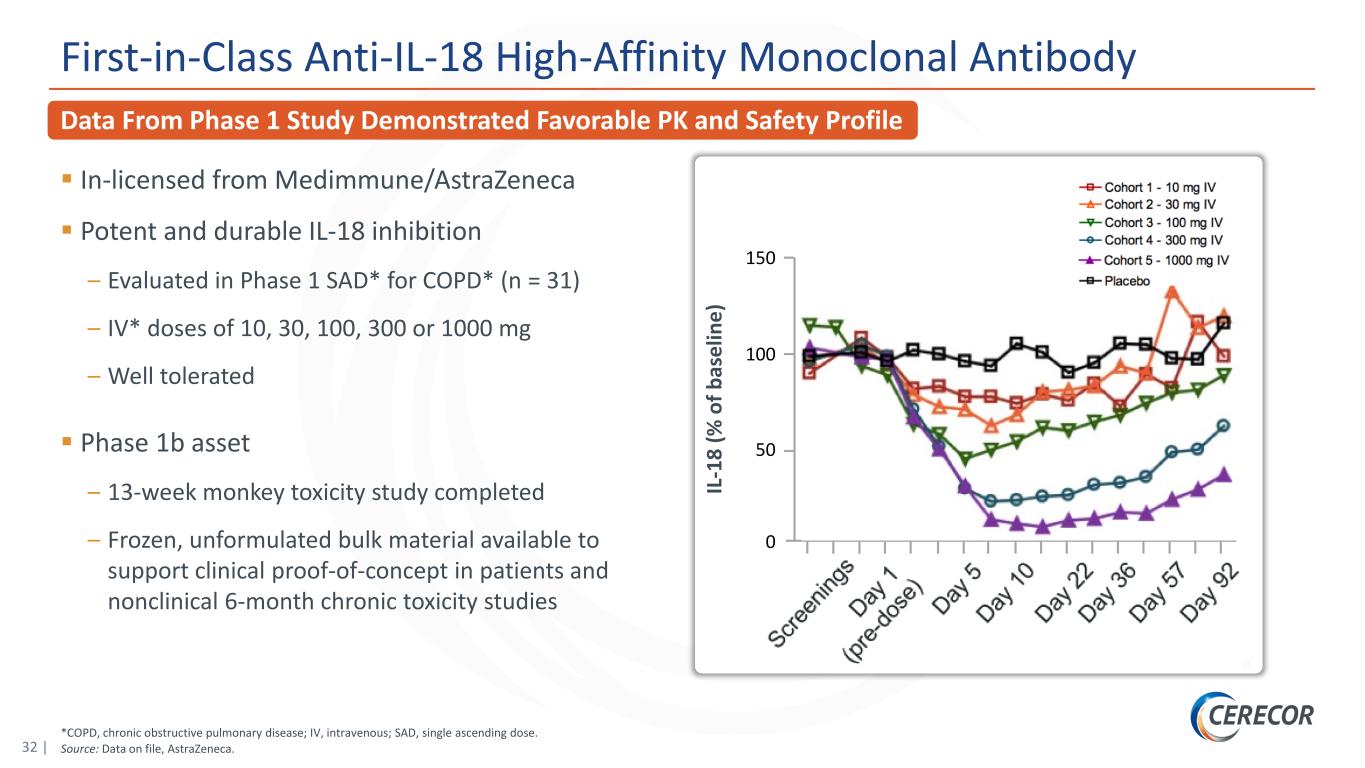
32 | *COPD, chronic obstructive pulmonary disease; IV, intravenous; SAD, single ascending dose. Source: Data on file, AstraZeneca. Data From Phase 1 Study Demonstrated Favorable PK and Safety Profile First-in-Class Anti-IL-18 High-Affinity Monoclonal Antibody In-licensed from Medimmune/AstraZeneca Potent and durable IL-18 inhibition – Evaluated in Phase 1 SAD* for COPD* (n = 31) – IV* doses of 10, 30, 100, 300 or 1000 mg – Well tolerated Phase 1b asset – 13-week monkey toxicity study completed – Frozen, unformulated bulk material available to support clinical proof-of-concept in patients and nonclinical 6-month chronic toxicity studies IL -1 8 (% o f b as el in e) 150 100 0 50
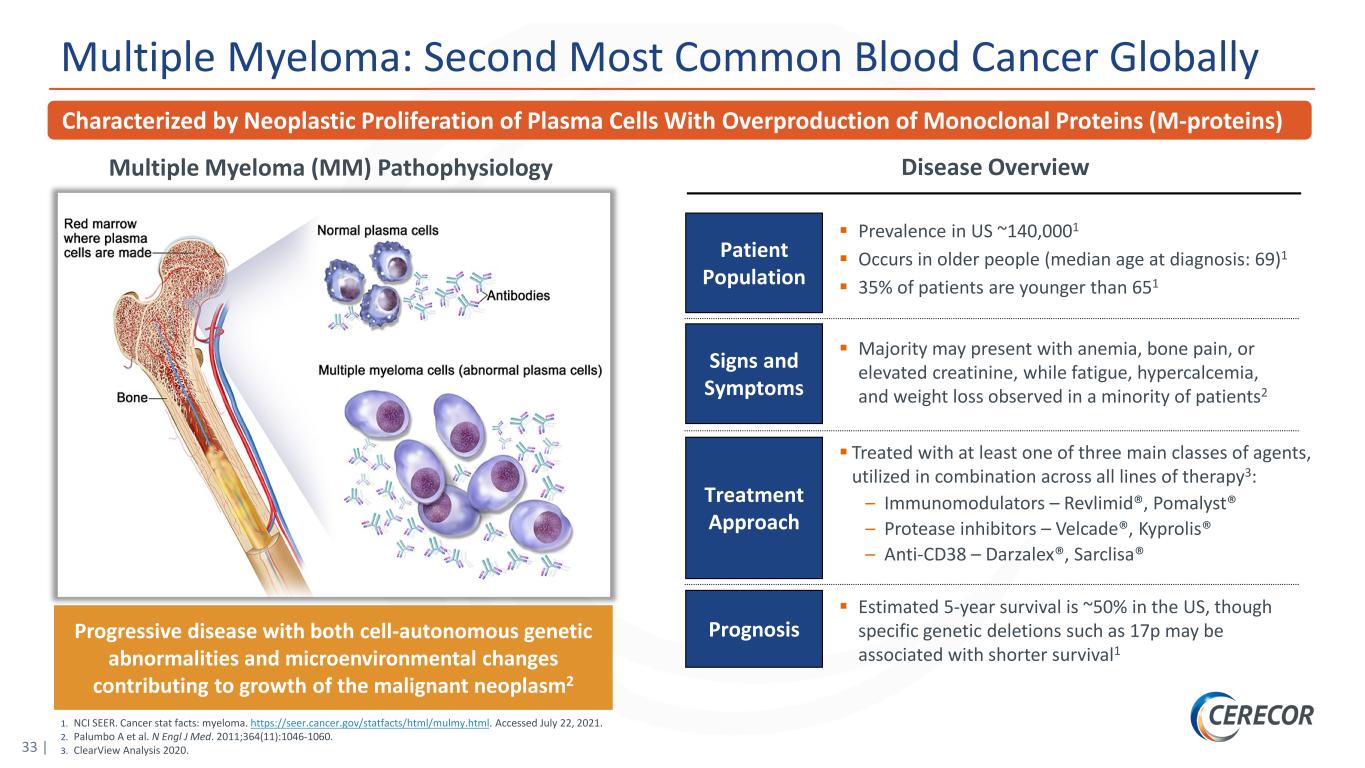
33 | 1. NCI SEER. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 22, 2021. 2. Palumbo A et al. N Engl J Med. 2011;364(11):1046-1060. 3. ClearView Analysis 2020. Multiple Myeloma: Second Most Common Blood Cancer Globally Disease OverviewMultiple Myeloma (MM) Pathophysiology Progressive disease with both cell-autonomous genetic abnormalities and microenvironmental changes contributing to growth of the malignant neoplasm2 Treatment Approach Majority may present with anemia, bone pain, or elevated creatinine, while fatigue, hypercalcemia, and weight loss observed in a minority of patients2 Signs and Symptoms Prognosis Estimated 5-year survival is ~50% in the US, though specific genetic deletions such as 17p may be associated with shorter survival1 Patient Population Prevalence in US ~140,0001 Occurs in older people (median age at diagnosis: 69)1 35% of patients are younger than 651 Treated with at least one of three main classes of agents, utilized in combination across all lines of therapy3: – Immunomodulators – Revlimid®, Pomalyst® – Protease inhibitors – Velcade®, Kyprolis® – Anti-CD38 – Darzalex®, Sarclisa® Characterized by Neoplastic Proliferation of Plasma Cells With Overproduction of Monoclonal Proteins (M-proteins)
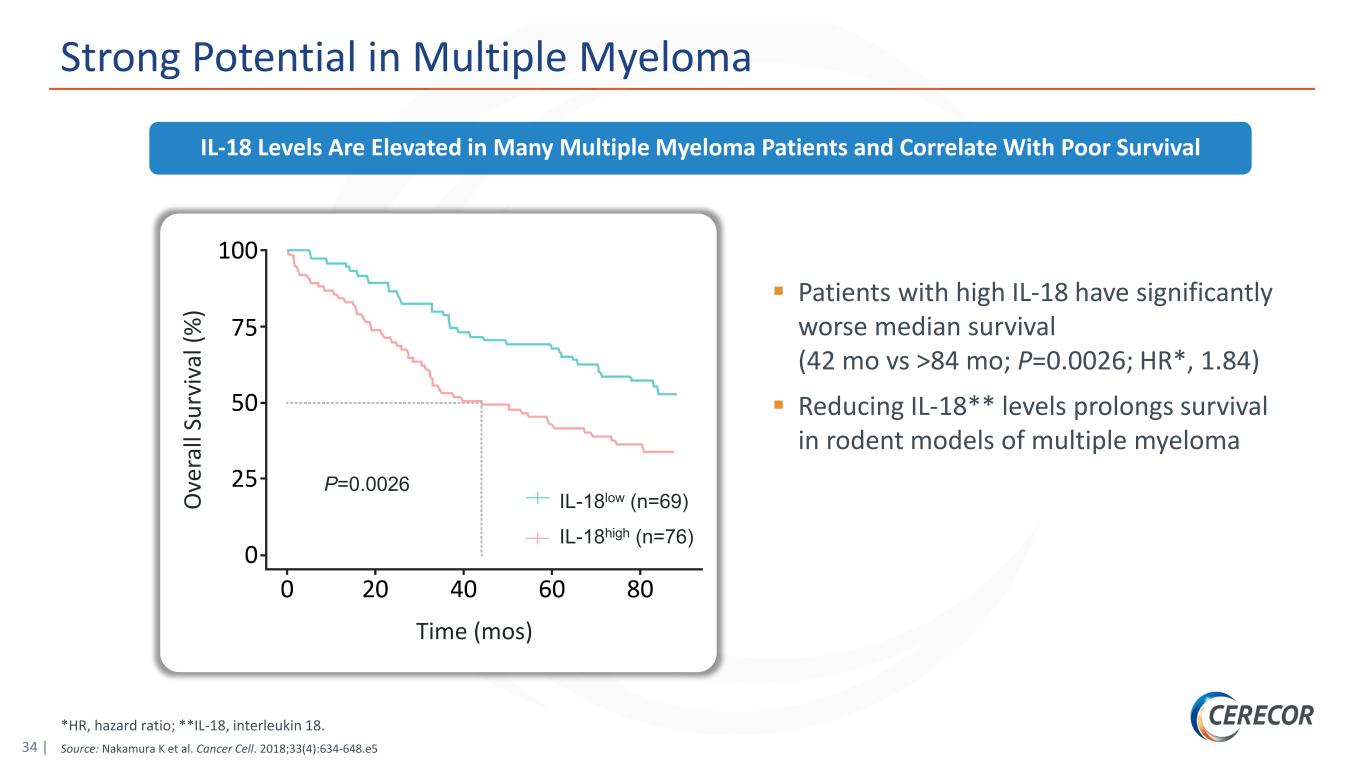
34 | *HR, hazard ratio; **IL-18, interleukin 18. Source: Nakamura K et al. Cancer Cell. 2018;33(4):634-648.e5 Strong Potential in Multiple Myeloma Patients with high IL-18 have significantly worse median survival (42 mo vs >84 mo; P=0.0026; HR*, 1.84) Reducing IL-18** levels prolongs survival in rodent models of multiple myeloma IL-18 Levels Are Elevated in Many Multiple Myeloma Patients and Correlate With Poor Survival IL-18low (n=69) IL-18high (n=76) P=0.0026 Time (mos) O ve ra ll Su rv iv al (% )
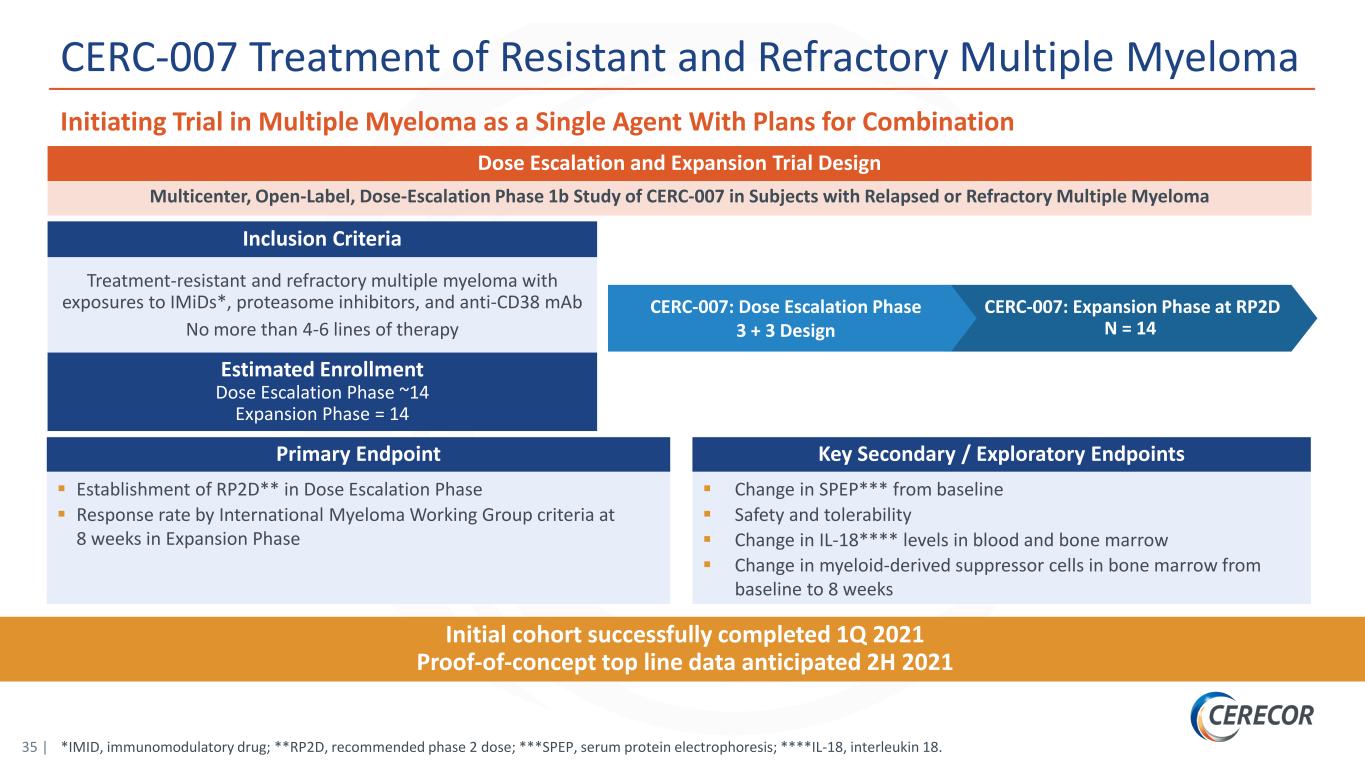
35 | *IMID, immunomodulatory drug; **RP2D, recommended phase 2 dose; ***SPEP, serum protein electrophoresis; ****IL-18, interleukin 18. CERC-007 Treatment of Resistant and Refractory Multiple Myeloma Initiating Trial in Multiple Myeloma as a Single Agent With Plans for Combination CERC-007: Expansion Phase at RP2D N = 14 Multicenter, Open-Label, Dose-Escalation Phase 1b Study of CERC-007 in Subjects with Relapsed or Refractory Multiple Myeloma Treatment-resistant and refractory multiple myeloma with exposures to IMiDs*, proteasome inhibitors, and anti-CD38 mAb No more than 4-6 lines of therapy Estimated Enrollment Dose Escalation Phase ~14 Expansion Phase = 14 Inclusion Criteria Dose Escalation and Expansion Trial Design Primary Endpoint Establishment of RP2D** in Dose Escalation Phase Response rate by International Myeloma Working Group criteria at 8 weeks in Expansion Phase Key Secondary / Exploratory Endpoints Change in SPEP*** from baseline Safety and tolerability Change in IL-18**** levels in blood and bone marrow Change in myeloid-derived suppressor cells in bone marrow from baseline to 8 weeks Initial cohort successfully completed 1Q 2021 Proof-of-concept top line data anticipated 2H 2021 CERC-007: Dose Escalation Phase 3 + 3 Design
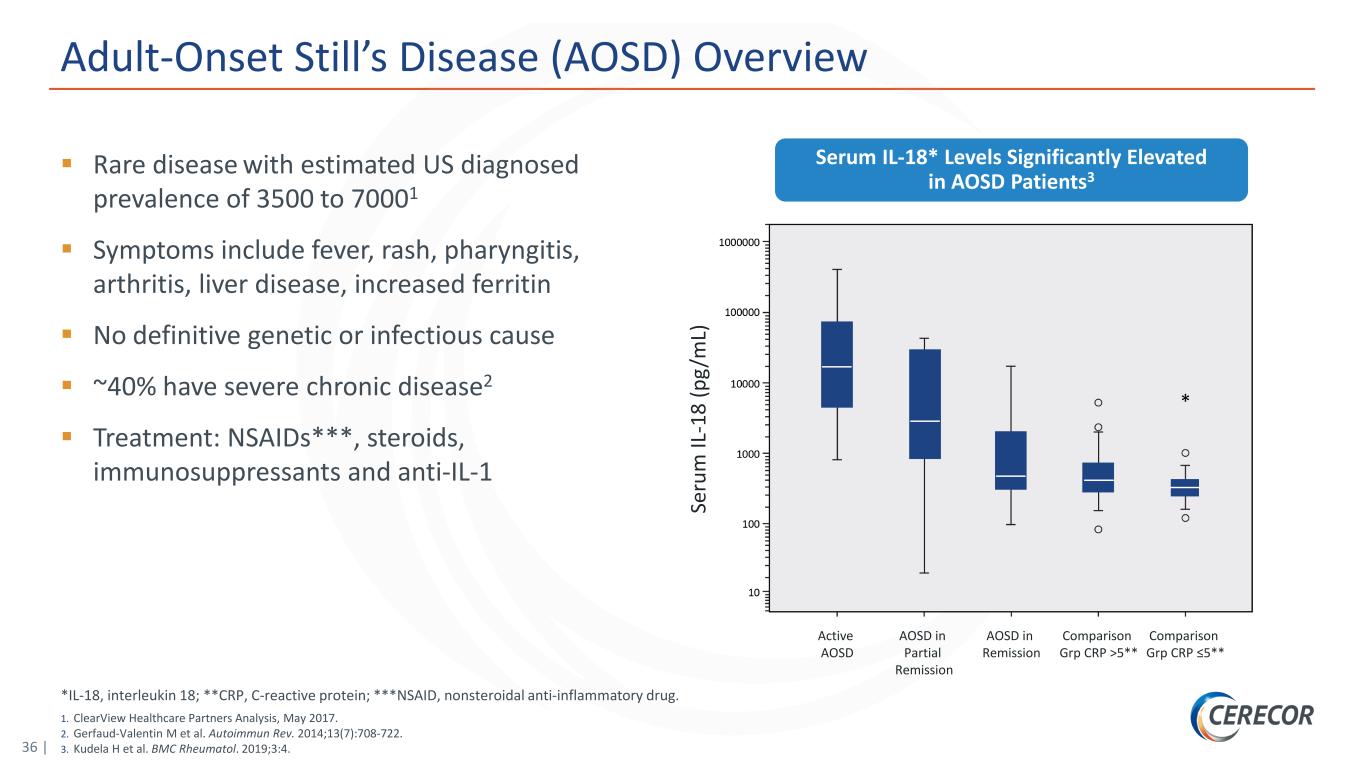
36 | *IL-18, interleukin 18; **CRP, C-reactive protein; ***NSAID, nonsteroidal anti-inflammatory drug. 1. ClearView Healthcare Partners Analysis, May 2017. 2. Gerfaud-Valentin M et al. Autoimmun Rev. 2014;13(7):708-722. 3. Kudela H et al. BMC Rheumatol. 2019;3:4. Adult-Onset Still’s Disease (AOSD) Overview Rare disease with estimated US diagnosed prevalence of 3500 to 70001 Symptoms include fever, rash, pharyngitis, arthritis, liver disease, increased ferritin No definitive genetic or infectious cause ~40% have severe chronic disease2 Treatment: NSAIDs***, steroids, immunosuppressants and anti-IL-1 Serum IL-18* Levels Significantly Elevated in AOSD Patients3 Se ru m IL -1 8 (p g/ m L) Active AOSD AOSD in Partial Remission AOSD in Remission Comparison Grp CRP >5** Comparison Grp CRP ≤5**
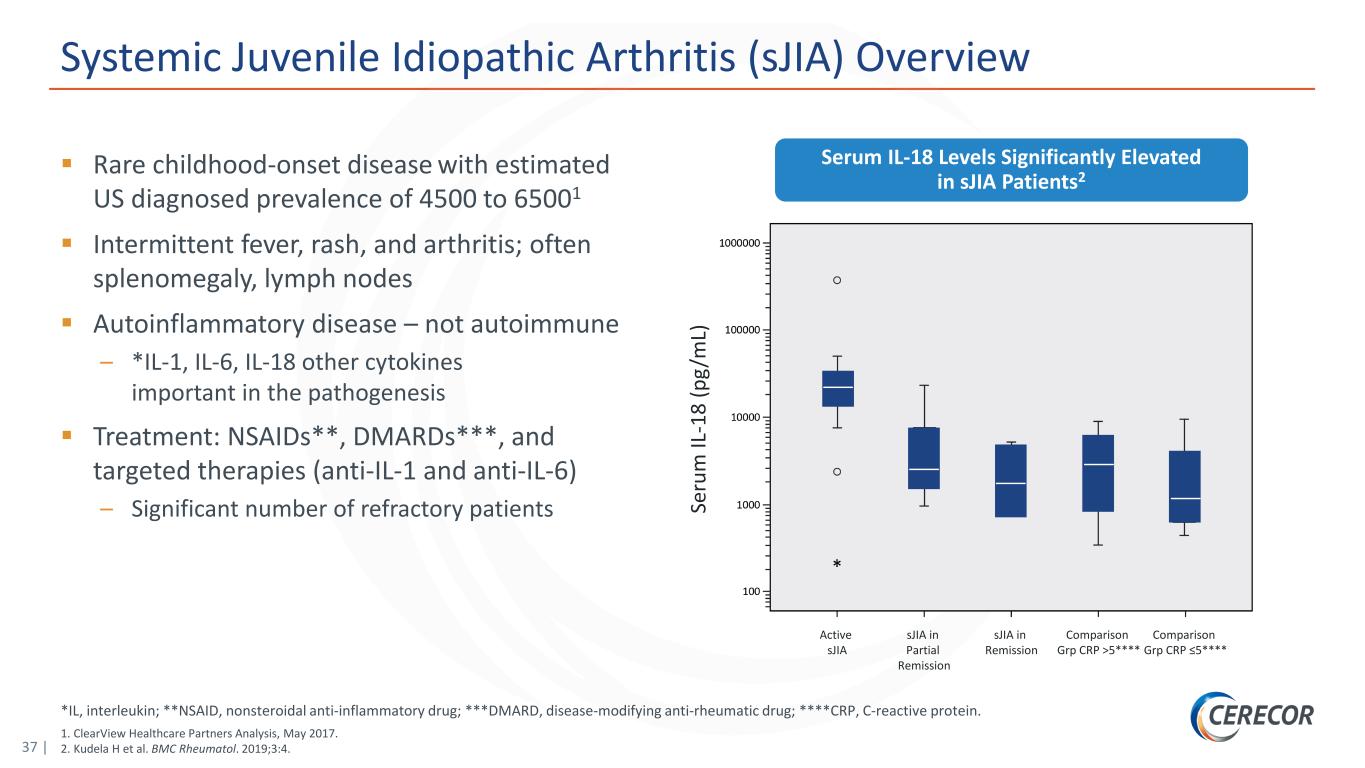
37 | *IL, interleukin; **NSAID, nonsteroidal anti-inflammatory drug; ***DMARD, disease-modifying anti-rheumatic drug; ****CRP, C-reactive protein. 1. ClearView Healthcare Partners Analysis, May 2017. 2. Kudela H et al. BMC Rheumatol. 2019;3:4. Systemic Juvenile Idiopathic Arthritis (sJIA) Overview Rare childhood-onset disease with estimated US diagnosed prevalence of 4500 to 65001 Intermittent fever, rash, and arthritis; often splenomegaly, lymph nodes Autoinflammatory disease – not autoimmune – *IL-1, IL-6, IL-18 other cytokines important in the pathogenesis Treatment: NSAIDs**, DMARDs***, and targeted therapies (anti-IL-1 and anti-IL-6) – Significant number of refractory patients Serum IL-18 Levels Significantly Elevated in sJIA Patients2 Se ru m IL -1 8 (p g/ m L) Active sJIA sJIA in Partial Remission sJIA in Remission Comparison Grp CRP >5**** Comparison Grp CRP ≤5****
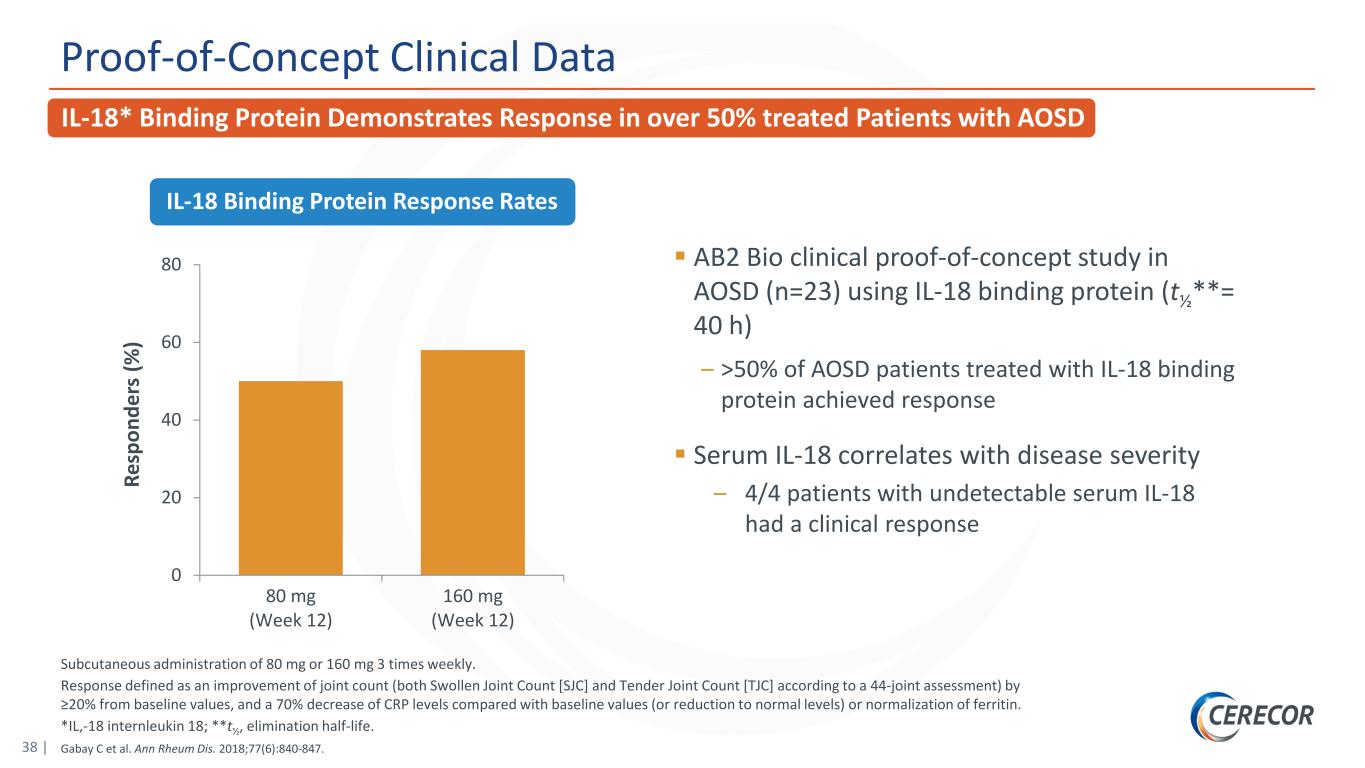
38 | Subcutaneous administration of 80 mg or 160 mg 3 times weekly. Response defined as an improvement of joint count (both Swollen Joint Count [SJC] and Tender Joint Count [TJC] according to a 44-joint assessment) by ≥20% from baseline values, and a 70% decrease of CRP levels compared with baseline values (or reduction to normal levels) or normalization of ferritin. *IL,-18 internleukin 18; **t½, elimination half-life. Gabay C et al. Ann Rheum Dis. 2018;77(6):840-847. Proof-of-Concept Clinical Data AB2 Bio clinical proof-of-concept study in AOSD (n=23) using IL-18 binding protein (t½**= 40 h) – >50% of AOSD patients treated with IL-18 binding protein achieved response Serum IL-18 correlates with disease severity – 4/4 patients with undetectable serum IL-18 had a clinical response 0 20 40 60 80 80 mg (Week 12) 160 mg (Week 12) Re sp on de rs (% ) IL-18 Binding Protein Response Rates IL-18* Binding Protein Demonstrates Response in over 50% treated Patients with AOSD
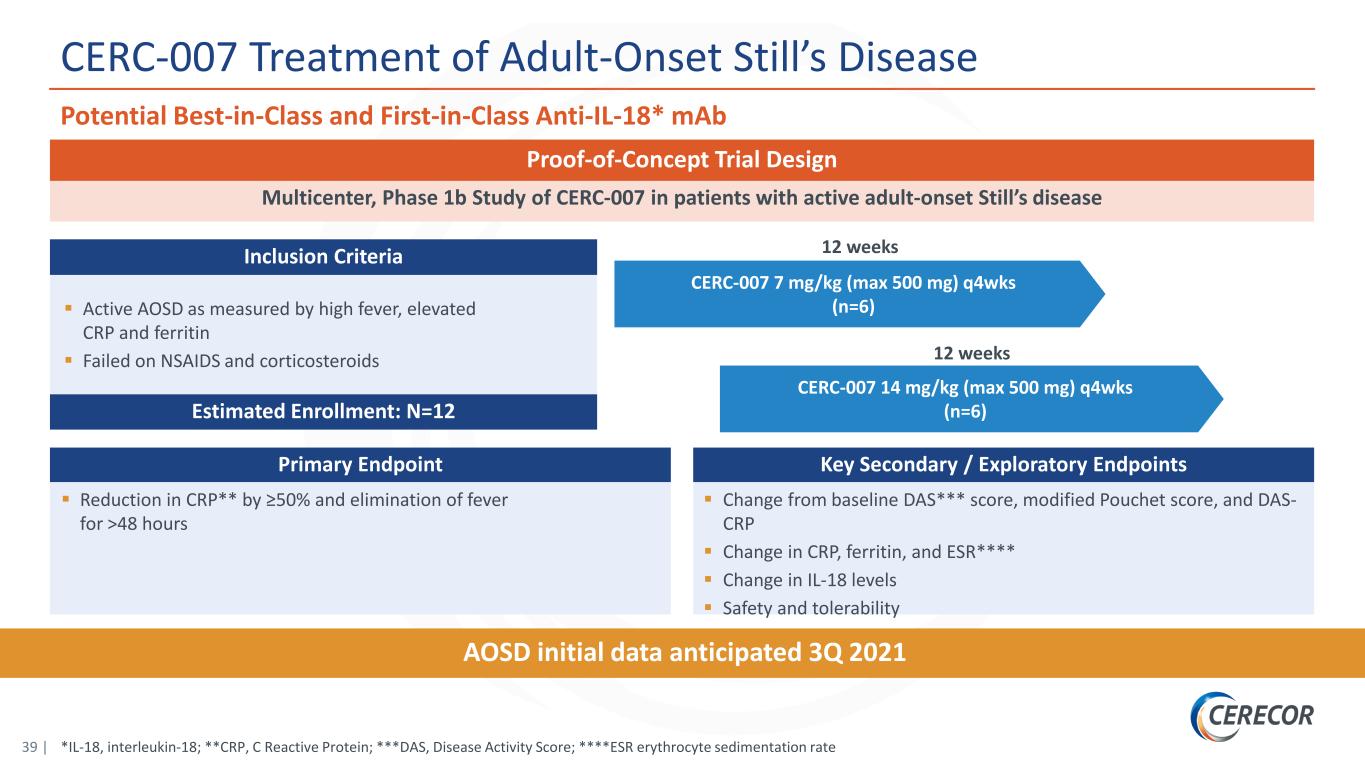
39 | CERC-007 7 mg/kg (max 500 mg) q4wks (n=6) *IL-18, interleukin-18; **CRP, C Reactive Protein; ***DAS, Disease Activity Score; ****ESR erythrocyte sedimentation rate CERC-007 Treatment of Adult-Onset Still’s Disease Potential Best-in-Class and First-in-Class Anti-IL-18* mAb 12 weeks AOSD initial data anticipated 3Q 2021 Multicenter, Phase 1b Study of CERC-007 in patients with active adult-onset Still’s disease Proof-of-Concept Trial Design Primary Endpoint Reduction in CRP** by ≥50% and elimination of fever for >48 hours Key Secondary / Exploratory Endpoints Change from baseline DAS*** score, modified Pouchet score, and DAS- CRP Change in CRP, ferritin, and ESR**** Change in IL-18 levels Safety and tolerability Active AOSD as measured by high fever, elevated CRP and ferritin Failed on NSAIDS and corticosteroids Estimated Enrollment: N=12 Inclusion Criteria CERC-007 14 mg/kg (max 500 mg) q4wks (n=6) 12 weeks

Phase 2-ready, dual mTORC 1/2 small molecule inhibitor for Complex Lymphatic Malformations CERC-006
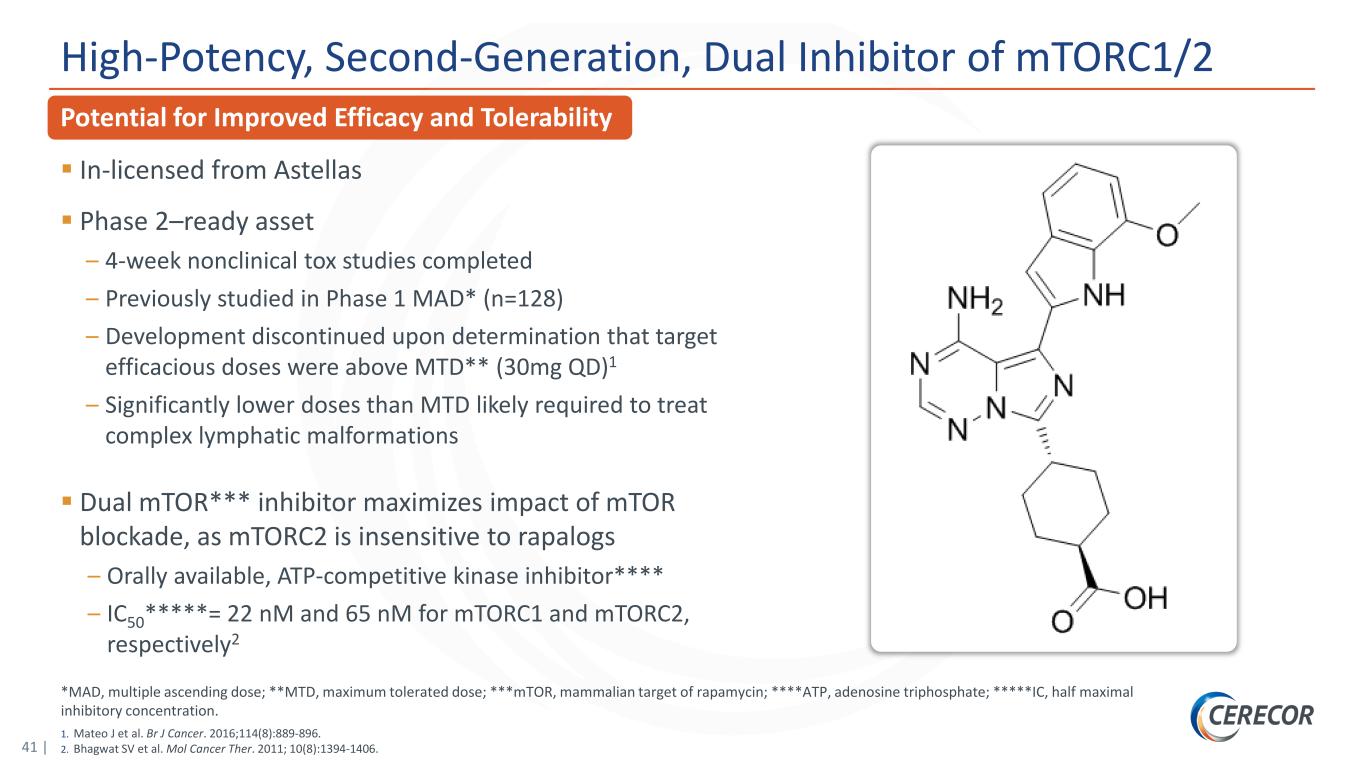
41 | *MAD, multiple ascending dose; **MTD, maximum tolerated dose; ***mTOR, mammalian target of rapamycin; ****ATP, adenosine triphosphate; *****IC, half maximal inhibitory concentration. 1. Mateo J et al. Br J Cancer. 2016;114(8):889-896. 2. Bhagwat SV et al. Mol Cancer Ther. 2011; 10(8):1394-1406. Potential for Improved Efficacy and Tolerability High-Potency, Second-Generation, Dual Inhibitor of mTORC1/2 In-licensed from Astellas Phase 2–ready asset – 4-week nonclinical tox studies completed – Previously studied in Phase 1 MAD* (n=128) – Development discontinued upon determination that target efficacious doses were above MTD** (30mg QD)1 – Significantly lower doses than MTD likely required to treat complex lymphatic malformations Dual mTOR*** inhibitor maximizes impact of mTOR blockade, as mTORC2 is insensitive to rapalogs – Orally available, ATP-competitive kinase inhibitor**** – IC50*****= 22 nM and 65 nM for mTORC1 and mTORC2, respectively2
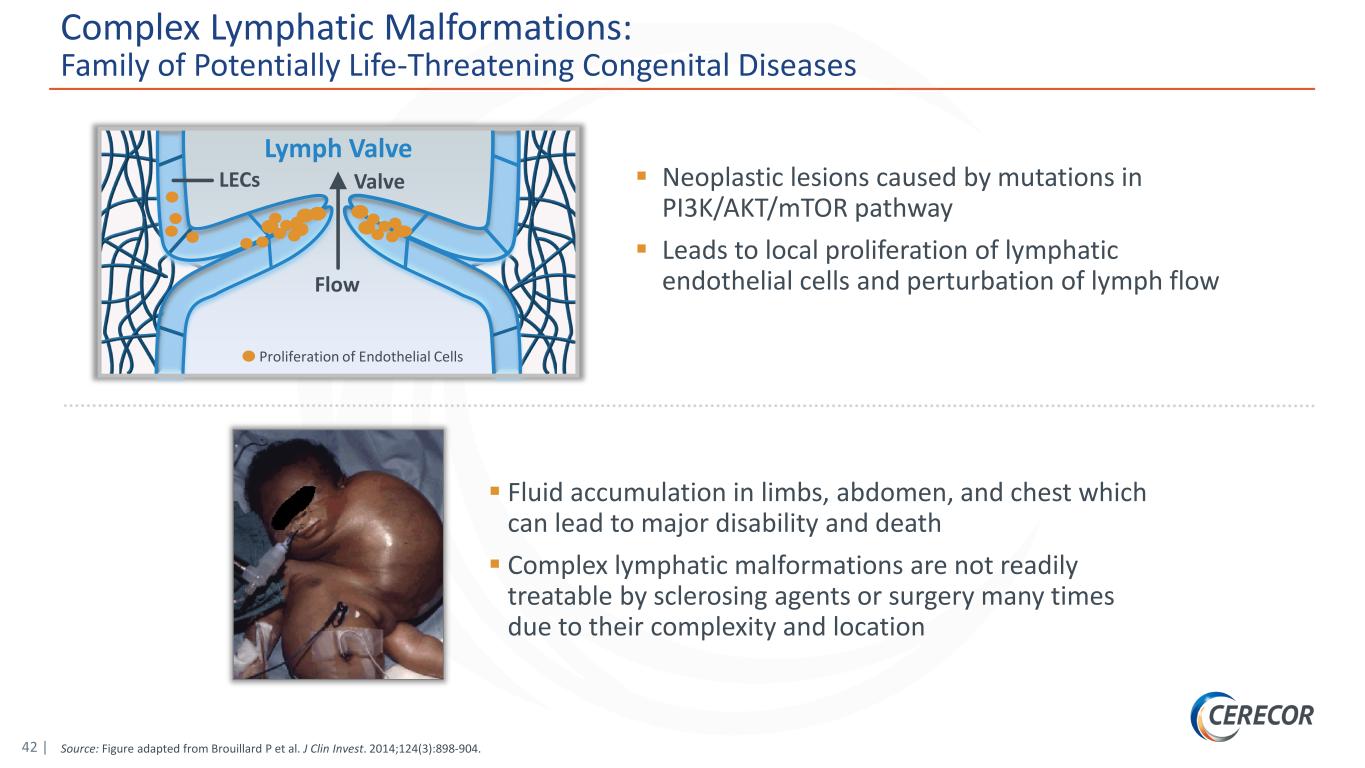
42 | Source: Figure adapted from Brouillard P et al. J Clin Invest. 2014;124(3):898-904. Complex Lymphatic Malformations: Family of Potentially Life-Threatening Congenital Diseases Neoplastic lesions caused by mutations in PI3K/AKT/mTOR pathway Leads to local proliferation of lymphatic endothelial cells and perturbation of lymph flow Fluid accumulation in limbs, abdomen, and chest which can lead to major disability and death Complex lymphatic malformations are not readily treatable by sclerosing agents or surgery many times due to their complexity and location Flow ValveLECs Lymph Valve Proliferation of Endothelial Cells
43 | Source: Adams DM et al. Pediatrics. 2016;137(2):e20153257. Open-Label Clinical Studies Support Efficacy; Use Is Limited by Tolerability Issues and Lack of FDA Approval Off-Label Use of mTOR Inhibitor Sirolimus in Lymphatic Malformations Phase 2 trial enrolled patients with complicated vascular anomalies – Enrolled patients with different subtypes of lymphatic malformations not controlled by previous medication, sclerotherapy, and/or surgery – Sirolimus was administered orally for 12 courses of 28 days each – 57 patients were evaluable for efficacy at the end of course 6, and 53 were evaluable at the end of course 12 Safety and tolerability profile leads to low compliance, requires frequent monitoring – Physicians reported that sirolimus caused high rates of stomatitis (~60%) – Sirolimus bears black box warning for immunosuppression and malignancies Overall Response 6-month (n=57) 12-month (n=53) Grade 2 or >AEs Complete response 0 0 • Blood/bone marrow (50%) • Gastrointestinal (55%) • Metabolic/laboratory (20%) • Infection (15%) Partial response 47 (83%) 45 (85%) Progressive disease 7 (12%) 8 (15%) Stable disease 3 (5%) 0
44 | *Pi3K, phosphatidylinositol 3-kinase; *AKT, protein kinase B. CERC-006 Treatment of Complex Lymphatic Malformations Dual mTOR Inhibitor to Modulate PI3K* and AKT** Activity CERC-006 two dose groups: 0.5 mg and 1mg twice daily 4-week treatment Initial data anticipated 3Q 2021 Multicenter, Phase 1b Study of CERC-006 in patients with complex lymphatic malformations Proposed Phase 1b Study Primary Endpoint Safety and tolerability of CERC-006 Key Secondary / Exploratory Endpoints PK and PD characteristics of CERC-006 Evidence of clinical signals utilizing quality of life and radiologic evaluation Clinical and laboratory safety assessments Selected biomarkers Adults 18-31 years with moderate to severe complex lymphatic malformations Estimated Enrollment: 10 Inclusion Criteria

Monosaccharide therapy for Congenital Disorders of Glycosylation (CDGs) CERC-800s
46 | 1. Wong et al. Genet Med. 2017;19(11):1226-1235. 2. Harms et al. Acta Paediatr. 2002;91(10):1065-1072. 3. Marquardt et al. Blood. 1991;94(12):3976-3985. Impaired Glycoprotein Production and Function Can Simply Be Restored With Substrate Supplementation Therapy Congenital Disorders of Glycosylation (CDGs): Life-Threatening, Ultra-Rare, Inborn Errors of Metabolism (IEMs) Glycosylation is essential for protein structure & function, particularly for circulating proteins and enzymes such as hormones and coagulation factors Currently approximately 150 CDGs identified Due to a genetic mutation, CDG patients lack the ability to synthesize functioning glycoproteins Life-threatening multi-system diseases: failure to thrive, developmental delay, hypotonia, neurologic abnormalities, hepatic disease, and coagulopathy Administration of therapeutic doses of specific monosaccharides targeted to specific CDGs can partially restore impaired glycoprotein production resulting in a meaningful clinical benefit – PGM1-CDG: D-galactose supplementation1 – MPI-CDG: D-mannose supplementation2 – LAD-II (SLC35C1-CDG): L-fucose supplementation3
47 | D-MannoseD-Galactose L-Fucose OHO OH OH HO OH OHO OH OH HO OH CERC-801 CERC-802 CERC-803 Accelerated pathway ✓ ✓ ✓ FDA ODD** ✓ ✓ ✓ Priority Review Voucher* ✓ ✓ ✓ Pivotal data anticipated 1Q 2022 2H 2021 2H 2021 *All three CERC-800 compounds granted Rare Pediatric Disease Designation prior to September 30, 2024; eligible for Priority Review Voucher upon approval. **Designation makes CERC-800 compound’s respective CDG indication eligible for 7-year orphan exclusivity upon approval. ***GMP, Good Manufacturing Practices; ODD, Orphan Drug Designation. Pharmaceutical Grade Treatments for CDGs Opportunity to Be the First FDA-Approved Drugs for CDGs Established therapeutic proof-of concept GMP*** manufacturing and FDA approval will ensure quality and consistency Potential for reimbursement OHO OH OH HO

Key Financial Information
49 | Financial & Investor Information Key Financial Highlights The following data is as of March 31, 2021 Outstanding common shares – 89.1M Fully diluted shares – 114.2M Average daily trading volume – 1M Cash – $38.3M NASDAQ: CERC

50 | Select Management Team Members Proven Track Record in Drug Development and Commercialization Michael Cola Chief Executive Officer • Former President of Specialty Pharmaceuticals, Shire plc • Senior management, AstraMerck and AstraZeneca plc Garry Neil, MD Chief Scientific Officer • Former Corporate VP of Science & Technology, Johnson & Johnson • Former Group President, Johnson & Johnson Pharmaceutical Research and Development • Former VP of Clinical Research, AstraZeneca plc and Merck KGaA H. Jeffrey Wilkins, MD Chief Medical Officer • Former VP, Worldwide Clinical Research, Inflammation/Oncology at Cephalon and SVP of Clinical Development with Ception Therapeutics • Former VP of Discovery Medicine for GSK’s Center of Excellence in External Drug Discovery

NASDAQ: CERC www.cerecor.com